Secondary physiological pneumonectomy to bronchial tuberculosis
Introduction
Tracheobronchial tuberculosis is a rare form of a respiratory tuberculosis, present in up to 90% of patients with bronchial stenosis (1). Bronchial tuberculosis is defined as an infection of the bronchial tree by Mycobacterium tuberculosis that can affect the mucous, submucous, muscularis and cartilage, progressing to lumen stenosis (2). The aim of this study is to present a case report of a patient with bronchial stenosis secondary to commitment bronchial tuberculosis.
Case presentation
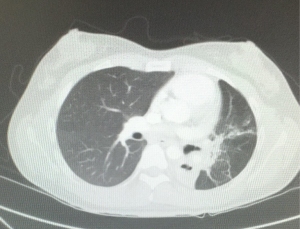
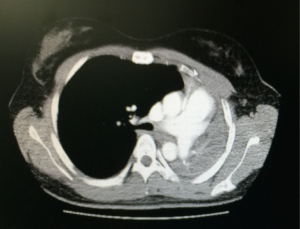
An 18-year-old female was with diagnosis of pulmonary tuberculosis by clinical findings, chest radiography and positive sputum for mycobacterium. She gets full treatment, with complete resolution of lung infection. A year later, due to hypoventilation on left side, we obtain a chest tomography that shows a decrease in the size of the left bronchus, bronchiectasis and basal consolidation (Figure 1), fibrobronchoscopy shows severe stenosis of the left main bronchus, unable to make bronchoplasty. Bronchoalveolar lavage was negative for mycobacterium tuberculosis. The patient was asymptomatic. A second scan of the chest was made three months after, which showed complete obstruction of the left main bronchus, with collapse of the left lung by severe fibrothorax, deviation of the mediastinum and trachea to the left, rising of the left hemidiaphragm and compensatory right hyperinflation (Figure 2). The control smears remain negative for acid-fast bacilli. Functional respiratory tests show a moderate restrictive pattern with an FVC of 69% and an FEV1 of 63%. Due to the absence of symptoms, proper functional class and high surgical risk, it was decided to have expectant management with pulmonary rehabilitation and strict clinical monitoring. The patient continues asymptomatic for 12 months of follow up without associated complications.
Discussion
There is no unanimous definition of tracheobronchial tuberculosis, it has been defined as “Mycobacterium tuberculosis infection of the tracheobronchial tree”, “a complication of a primary progressive pulmonary tuberculosis” (1).
Risk factors for the development of the tracheobronchial tuberculosis are: female gender, a prolonged evolution of pulmonary tuberculosis (>4 weeks) and the absence of previous tuberculosis infection (3).
The main mechanisms of development include: direct infection of the bronchial mucosa, adjacent infiltration of pulmonary lesions, ruptured of lymph nodes around the bronchus and hematogenous dissemination (4).
Clinically it could have an asymptomatic presentation, an insidious course that can mimic lung cancer or acute course with bronchial obstruction and acute respiratory infections (2). Only a minority of patients (13%) have no active lesions of pulmonary tuberculosis (4), with normal chest radiograph in 10–20% of patients (2).
It is reported in literature the high incidence of tracheobronchial involvement in patients with pulmonary cavitated lesions possibly secondary to the increased exposure of the airways to the bacillus, however this association is variable in studies (3).
In the fiberoptic bronchoscopy a wide range of injuries are described (5). Such injuries are related to the positive cultures, the favorable response to anti-tuberculous treatment and the development of bronchial stenosis (2).
The discovery of mycobacterium tuberculosis in sputum ranges from 16–53%, with other methods such as PCR amplification of DNA or RNA as complementary tests (1).
Infection control and prevention of stenosis are the mainstay of the treatment in the tracheobronchial tuberculosis; its outcome will depend on the degree of commitment and length of lesions after treatment (3,5).
Most patients respond favorably to anti tuberculosis therapy and although the incidence of decreasing the bronchial lumen is initially high, only 30% or less of the patients develop moderate to severe bronchostenosis (3).
In our case, the patient has clear risk factors for the development of a bronchial infection, with subsequent stenosis of the airways. It has a remarkably rapid evolution, with an accelerated progression to a complete pulmonary stenosis with a complete lung collapse, configuring into a secondary physiological pneumonectomy, with an asymptomatic evolution. In the existing literature there are few reported cases of physiological pneumonectomy secondary to a bronchial tuberculosis infection, as the one presented in this case. Surgical treatment for this patient has a high surgical risk, high risk of bleeding and bronchopleural fistula. The benefit of surgery on the other hand is difficult to prove because in this specific case the lung is abolished of any physiological function of gas exchange. Before you can draw specific conclusions, one should expect the long-term evolution to define the benefits of the expectant versus surgical management in this rare group of patients with severe bronchial stenosis and auto pneumonectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Casali L, Crapa ME. Endobronchial Tubercolosis: a peculiar feature of TB often underdiagnosed. Multidiscip Respir Med 2012;7:35. [Crossref] [PubMed]
- Xue Q, Wang N, Xue X, et al. Endobronchial tuberculosis: an overview. Eur J Clin Microbiol Infect Dis 2011;30:1039-44. [Crossref] [PubMed]
- Jung SS, Park HS, Kim JO, et al. Incidence and clinical predictors of endobronchial tuberculosis in patients with pulmonary tuberculosis. Respirology 2015;20:488-95. [Crossref] [PubMed]
- Kurasawa T, Kuze F, Kawai M, et al. Diagnosis and management of endobronchial tuberculosis. Intern Med 1992;31:593-8. [Crossref] [PubMed]
- Smith LS, Schillaci RF, Sarlin RF., et al. Endobronchial tuberculosis. Serial fiberoptic bronchoscopy and natural history. Chest 1987;91:644-7. [Crossref] [PubMed]
Cite this article as: Zapata González RA, Salinas Parra C, Torres Rincón RA. Secondary physiological pneumonectomy to bronchial tuberculosis. Ann Res Hosp 2017;1:12.