
Resolution of a respiratory failure due to massive chronic pericardial effusion with a pericardial window: the simplest is the best
Introduction
The pericardium fixes the heart to the mediastinum, protects against infection and provides lubrication for the heart. The average pericardial sac contains 10–50 mL of pericardial fluid (plasma ultrafiltrate) that acts as a lubricant between the pericardial layers. Any pathological process causes an inflammation with the possibility of increased production of pericardial fluid (exudate). An alternative mechanism of accumulation of fluid may be the decreased reabsorption due to a general increase in systemic venous pressure because of congestive heart failure or pulmonary hypertension (transudate). A considerable proportion of patients with pericardial effusion (PE) are asymptomatic, and PE constitutes an incidental and unexpected finding on roentgenogram or echocardiogram performed for other reasons. A slow increase of pericardial fluid allows the collection of a large amounts in weeks before a significant increase in pericardial pressure causes symptoms and signs. Although echocardiography remains the primary diagnostic tool for the study of pericardial, computed tomography (CT) and magnetic resonance provide a larger field of view, allowing the detection of loculated PE, pericardial thickening, and masses, as well as associated chest abnormalities. A severe effusion without cardiac tamponade and inflammatory signs are usually related to a chronic idiopathic aetiology (1).
Case presentation
A 79-year-old male patient was admitted to our hospital from a county hospital with a diagnosis of bilateral pneumonia complicated by septic shock and congestive heart failure. Two months earlier, he was hospitalised for a respiratory failure due to a congestive heart failure. Moreover, in the past 5 years, he was hospitalised three times for comparable symptoms. His past medical history includes 7 years of domiciliary oxygen therapy for restrictive COPD respiratory failure with peribronchial fibrosis, chronic cor pulmonale, past tobacco consumption, chronic atrial fibrillation treated with old type oral anticoagulant, ischemic/hypertensive heart disease, idiopathic chronic PE, chronic kidney failure, uncomplicated abdominal aortic aneurysm and thyroidectomy. The chest CT scan showed a remarkable PE (thickness over 10 cm) and bilateral lung inflammation (Figure 1). Echocardiogram showed a hypertensive heart disease with a proper left ventricular systolic function, slight pulmonary hypertension, severe ubiquitous PE without any hemodynamic problem and sharp right and left atrial dilatation. Once the patient’s conditions were stabilised, and the broad spectrum antibiotic therapy was administered, the patient’s general clinical conditions gradually improved. Therefore, a multidisciplinary meeting decided for a pericardial window after resolution of pneumonia. After 10 days, another chest CT scan, which confirmed the compression of the parenchyma with overwhelming of the carina and the main bronchi due to the large pericardial sac (Figure 2). Although the PE led to no hemodynamic problems, we decided to carry out a subxiphoid pericardial window to reduce the volume of the pericardial sac. Considering the comorbidities, we opted for local anaesthesia and mild sedation instead of general anaesthesia. During the surgical intervention, 2,150 mL of serum-blood fluid were drained in addition to the fluid filtered directly into the peritoneal cavity. During the first postoperative day, additional 1,000 mL were extracted through the pericardial drainage. The patient reported a remarkable subjective improvement. The histological examination of the pericardium showed a mild chronic lymphoplasmacellular perivascular inflammation. The CT scan 5 days after the pericardial window confirmed the decompression of the carina, the re-expansion of the left lung, the reduction of the pulmonary infiltrates, and a mild pneumopericardium (Figure 3). The echocardiogram confirmed all the previous reports, except for the PE, confirming that was not necessary from a hemodynamic point of view. One week after the surgical intervention, the pO2 values steadily amounted to about 70 mmHg; therefore, the oxygen therapy was suspended. After 3 months from the pericardial window, pO2 was 77 mmHg, forced expiration volume in the first second corresponded was 85% of the predicted value (it was 55% before surgery), and ventilatory capacity reached 81% of the predicted value (it was 52% before surgery). The diffusion capacity of the lung for carbon monoxide, never tested before, revealed a severe deficit (32%) of gaseous exchanges. The weight loss globally amounted to 13 kg (from 118 to 105 kg). The echocardiogram showed a mild PE mainly posterior, with fibrin deposits; the ejection fraction was normal, and the pulmonary artery pressure had still slightly increased. The chest CT scan confirmed a residual pericardial posterior and right effusion (maximum thickness of 2.7 cm). The healthy status returned normal, and the patient fully recovered his autonomy.
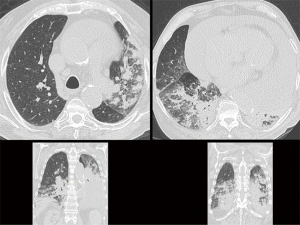
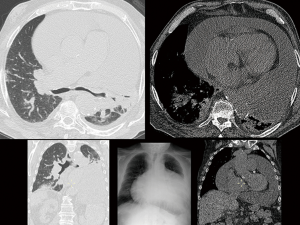
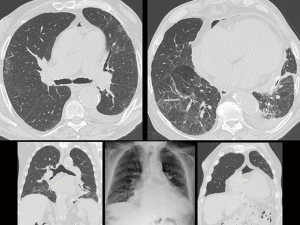
Discussion
Chronic PE—which is referred to as such when it is present for at least 3 months—is almost always associated with the presence/absence of cardiac tamponade. Data showed that 30–35% of chronic PE leads to cardiac tamponade (2). The PE is occasionally diagnosed, and in Western Countries, the percentage of idiopathic PE amounts to 50% of the cases (1). Multiple aetiologies, the various mechanisms that contribute to fluid accumulation, a plethora of treatment options that have yet to be class ranked and a frail patient cohort often compound to pose significant management challenges with a substantial financial burden.
Currently available clinical guidance was published in 2015 (1). Since then, it has been an expansion in our understanding of this subject and our pharmacological and interventional armamentarium. A search of the largest clinical trials registry (3) (clinicaltrials.gov) in July 2017 revealed only seven ongoing trials dealing with the topic of PE at the time of writing this article. Clinical practice is thought to be resultantly highly variable, although no published data previously existed to support this claim. Although many articles specifically address PE in patients with cancer, there have been no randomised controlled trials or prospective intervention trials (4). Treatment of PE secondary to cancer disease requires thought of the patient’s prognosis, the availability of expertise (5).
There are no effective drugs for the treatment of remote PE in the absence of evidence of pericarditis (5). Therefore, the pericardiocentesis and, in the case of relapse, the pericardiectomy or the less invasive pericardial window should be considered for the treatment of PE (6).
In the absence of any cardiac tamponade or known aetiology, the diagnostic/treatment path of chronic PE is not definitive yet. PE should be suspected in any patient with a malignancy and any of the following symptoms: dyspnoea or pleuritic chest pain, new radiographic cardiomegaly without pulmonary congestion, unexplained persistent fever, the presence of an isolated left pleural effusion, or hemodynamic deterioration of unknown aetiology. The most common malignancies causing PE are breast and lung cancer, followed by Hodgkin lymphoma, non-Hodgkin lymphoma, and leukaemia. Consequently, it is of particular importance for advanced practitioners to have a high index of suspicion for a patient with one of these malignancies presenting with shortness of breath. Radiation-induced PE may occur during the radiation therapy itself or up to 20 years after treatment, and a history of thoracic radiotherapy should further increase the suspicion of PE (7).
A patient with an average PE may be minimally symptomatic and may have no specific physical exam findings and a normal ECG. If a PE is within the differential diagnosis for the reasons outlined here, echocardiography is the most precise and clinically relevant diagnostic tool, as it can provide evidence of cardiac compromise before the development of overt tamponade. In slow PE without hemodynamic compromise, systemic chemotherapy is often a better option than intervention. Any patient who develops symptomatic tamponade from a malignant PE needs intervention for the effusion, preferably with pericardiocentesis followed by systemic chemotherapy. For recurrent symptomatic malignant PE, surgical intervention may, after the initial post-surgical recovery period, improve the quality of life and reduce hospital stays.
In conclusion, in the presence of restrictive or obstructive respiratory disease associated with chronic PE, the volume of the pericardial sac should always be considered, together with the consequences possibly arising from the compression of lungs and airways. Our case report still evidenced the poor adoption of the existing clinical guidance, as well as the need for simpler clinical recommendations for a better-informed practice. As stated in the first century of the last millennium in Ockham’s razor by William of Ockham, more straightforward statements are preferable to more complex ones since more testable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-64. [Crossref] [PubMed]
- Sagristà-Sauleda J, Angel J, Permanyer-Miralda G, et al. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med 1999;341:2054-9. [Crossref] [PubMed]
- Huser V, Cimino JJ. Evaluating adherence to the International Committee of Medical Journal Editors' policy of mandatory, timely clinical trial registration. J Am Med Inform Assoc 2013;20:e169-74. [Crossref] [PubMed]
- Burazor I, Imazio M, Markel G, et al. Malignant pericardial effusion. Cardiology 2013;124:224-32. [Crossref] [PubMed]
- Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J 2013;34:1186-97. [Crossref] [PubMed]
- Imazio M, Mayosi BM, Brucato A, et al. Triage and management of pericardial effusion. J Cardiovasc Med (Hagerstown) 2010;11:928-35. [Crossref] [PubMed]
- Petrofsky M.. Management of Malignant Pericardial Effusion. J Adv Pract Oncol 2014;5:281-9. [PubMed]
Cite this article as: Bonfanti B, Bertolaccini L, Pavesi P, Detotto E, Parini S, Solli P, Forti Parri SN. Resolution of a respiratory failure due to massive chronic pericardial effusion with a pericardial window: the simplest is the best. Ann Res Hosp 2017;1:34.


