
Infiltrative squamous cell carcinoma of the gallbladder: a unique approach to surgical palliation
Introduction
Gallbladder cancer (GBC) is the most common malignancy of the biliary tract worldwide. However, it remains to be quite a rarity, with the finding of a squamous carcinoma being an even rarer occurrence (1). The incidence of a pure squamous cell carcinoma (SCC) of the gallbladder accounts for roughly 1% of all reported gallbladder malignancies (1). Though numerous theories of the histological progression of squamous carcinoma have been proposed it is far from understood (2). Regardless of histology GBC has an overall poor prognosis with survival rarely seen past a few months (1). In those with squamous cell pathology the prognosis is even worse and may be related to the enhanced proliferative capability and tendency of invading local tissues (1,3).
As of today, the only effective and curative modality available in dealing with GBC is surgical resection (4). The extent of resection necessary to achieve these curative results ultimately depends on the extent of invasion through the gallbladder and its surrounding structures (5). Here we present a case of SCC of the gallbladder with direct local invasion into surrounding structures, specifically the duodenum. This case highlights the importance of palliative surgical care when faced with this pathology.
Case presentation
A 64-year-old woman presented with a 3-week history of nausea and vomiting. At this visit the patient also reported anorexia, intermittent subjective fevers, and a 15-lb weight loss over the last 3 weeks. On physical examination, the patient exhibited neither abdominal tenderness nor a Murphy’s sign. An abdominal ultrasound was performed revealing gallstones and sludging. An abdominal X-ray was also ordered showing a calcified mass in the right upper quadrant, originally read as a renal mass by radiology. Due to dehydration and inability to tolerate oral intake the patient was admitted to the hospital. Due to concerns for malignancy, on admission a CT scan of the abdomen was ordered with free air identified in the gallbladder fossa, consistent with perforation and a mass effect compressing the second portion of the duodenum (Figure 1).
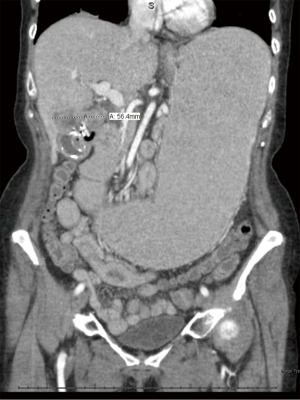
The patient subsequently underwent a laparoscopic cholecystectomy. Intraoperatively, the neck of the gallbladder was noted to be intrahepatic in position and significantly adhered to the duodenum. Biopsies of the fundus and intrahepatic back wall were sent for pathological evaluation, with pure SCC lacking any glandular component identified (Figures 2–3). Intraoperatively, it was decided to proceed with a laparoscopic gastrojejunostomy due to concerns with possible gastric outlet obstruction in the future. After the operation, the patient remained stable and was subsequently discharged on post-operative day 4 with referral to oncology and hepatobiliary surgery. Patient underwent subsequent chemotherapy treatment with Capecitabine and is presently alive 2 and a half years after her original surgery.
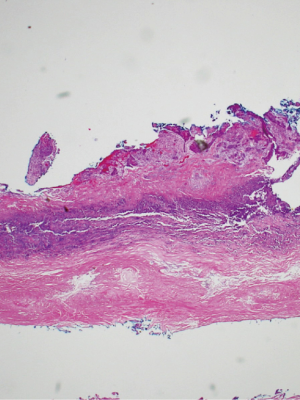
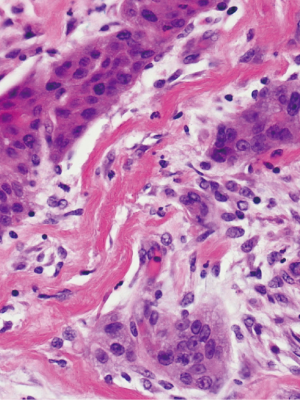
Discussion
The incidence of GBC of the squamous variant is a rare malignancy with a poor prognosis. Roa et al. reviewed 606 GBC cases and found those with pure squamous and adenosquamous variants to have a significantly worse outcome in comparison to those without squamous histology (median 4 vs. 12 months, P<0.003) (1). This can be attributed to SCC’s rapidly growing and locally aggressive nature (1,6,7). Nishihara et al. found that areas of SCC had a significantly greater proliferative capacity in comparison to pure adenocarcinoma (AC) (mean surface area: SCC =20.55% vs. AC =11.40%, P=0.0029) (3). In relation, the dismal survival rates seen with these tumors has also been found to correlate with tumor stage (4). A study from Memorial Sloan-Kettering attributed this correlation due to the increased likelihood of residual disease as the tumor stage escalates (residual disease present by stage after resection: T1 =50%, T2 =61%, T3 =85%, T4 =100%, P=0.0001) (4).
The pathogenesis of squamous carcinoma localized to the gallbladder has been postulated, but a consensus has yet to be reached. Some experts believe the pathogenesis behind squamous metaplasia may be similar to the neoplastic processes seen with SCC in other organ systems (8). Another mechanism proposed is that the squamous differentiation may be derived from a step-wise progression from an underlying AC (3). Regardless of the pathogenesis this case demonstrated a pure squamous cell malignancy without an underlying component of AC, a rare variant in this already rare malignancy.
Given the historical track record in reported cases of SCC of the gallbladder the presentation seen in our case is also quite unique. The symptoms experienced by the patient are noteworthy as she presented in an atypical fashion. Our patient reported anorexia, weight loss and the absence of any abdominal pain or tenderness over the span of 3 weeks. Additionally, upon further work up free air was appreciated in the gallbladder fossa concerning for perforation. Song et al., observed a trend in symptoms experienced by those with GBC finding abdominal pain, weight loss and jaundice the most commonly reported in SCC and AC (abdominal pain: 73.5% vs. 75.9%, weight loss: 38.2% vs. 31.6%, jaundice: 20.6% vs. 26%), however these were not statistically significant between the two variants (7).
The pattern of local invasion in our case further highlights the peculiarity surrounding this malignancy. Our patient was found to have infiltration into the duodenum, as a large majority of cases report local extension into the liver and gallbladder fossa this sequela is again a rarity (6,7,9). In relation, Karasawa et al. reported two cases of “pure” heavily keratinized SCC of the gallbladder, however these were encapsulated and confined to the gallbladder (8). Mghirbi et al. also reported a case of SCC of the gallbladder, however in this case the patient sustained local invasion to the right colon (10).
The standard of care regarding GBC treatment remains ill defined. Presently, a complete R0 resection remains the optimal method for curative treatment (4,7). The use of a R0 surgical resection for SCC variants showed significantly better 1-year survival results in comparison to those whom underwent an R1 or R2 resection (30% vs. 0%; P=0.025) (7). This study however, also found that those with SCC whom had undergone an R0 resection had a worse 1-year survival rate when compared to AC with a R0 resection (30% versus 69.3%; P=0.016) (7). In cases where an R0 resection cannot be obtained, resection followed by adjuvant chemotherapy or radiotherapy have been proposed with minor improvement in outcomes, however the data is limited (4). The patient in our case presented with an infiltrating mass abutting the duodenum with concerns for acute perforation. In addition, to a cholecystectomy a gastrojejunostomy was performed due to anticipated future obstruction of the gastric outlet. In reviewing literature no distinct cases have been reported where palliative bypassing of an infiltrating lesion from GBC into the duodenum was performed. The patient in our case underwent this very palliative by-passing, sustained an additional 2.5 years of life with additional chemotherapy postoperatively and presently remains alive. This survival length is unprecedented, as median survival of patients with stage IV disease has been reported to be 5.8 months (95% CI: 12.4–18.4) (4). And although an R0 resection was not attainable in this situation, the use of this technique should be investigated further as a palliative method for dealing with un-resectable disease in an obstructive pattern.
Conclusions
Literature surrounding GBC of the SCC variant is exceedingly rare. With minimal cases reported each instance and encounter with this malignancy allows the medical community to expand knowledge and improve management. The poor prognosis seen with this malignancy highlights the need for further modalities to enhance cure rates. However, presently the palliative surgical management of un-resectable disease remains the main option. Our case depicts an atypical presentation in a rare variant of GBC, infiltrating a less common location. The intra-operative management utilized in this case further highlights additional options for surgical palliation, via use of a gastrojejunostomy, which has not been reported in treating patients with GBC whom have had prolonged survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient in this case report has previously been consented and throughout the entirety of treatment has been maintained anonymous.
References
- Roa JC, Tapia O, Cakir A, et al. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol 2011;24:1069-78. [Crossref] [PubMed]
- Hosseinzadeh M, Shokripur M, Salahi H. Primary pure squamous cell carcinoma of gallbladder presenting as acute cholecystitis. Iran J Med Sci 2012;37:271-3. [PubMed]
- Nishihara K, Nagai E, Izumi Y, et al. Adenosquamous carcinoma of the gallbladder: a clinicopathological, immunohistochemical and flow-cytometric study of twenty cases. Jpn J Cancer Res 1994;85:389-99. [Crossref] [PubMed]
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- de Aretxabala X, Roa I, Burgos L, et al. Gallbladder cancer: an analysis of a series of 139 patients with invasion restricted to the subserosal layer. J Gastrointest Surg 2006;10:186-92. [Crossref] [PubMed]
- Chan KM, Yu MC, Lee WC, et al. Adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol 2007;95:129-34. [Crossref] [PubMed]
- Song HW, Chen C, Shen HX, et al. Squamous/adenosquamous carcinoma of the gallbladder: Analysis of 34 cases and comparison of clinicopathologic features and surgical outcomes with adenocarcinoma. J Surg Oncol 2015;112:677-80. [Crossref] [PubMed]
- Karasawa T, Itoh K, Komukai M, et al. Squamous cell carcinoma of gallbladder--report of two cases and review of literature. Acta Pathol Jpn 1981;31:299-308. [PubMed]
- Gulwani HV, Gupta S, Kaur S. Incidental detection of carcinoma gall bladder in laparoscopic cholecystectomy specimens: a thirteen year study of 23 cases and literature review. Indian J Surg Oncol 2015;6:30-5. [Crossref] [PubMed]
- Mghirbi F, Ayadi M, Karray W, et al. Squamous cell carcinoma of the gallbladder. Transl Gastroenterol Hepatol 2016;1:78. [Crossref] [PubMed]
Cite this article as: Trujillo CN, Fowler AB, Shapera EA, Theerman IL, Tessier DJ. Infiltrative squamous cell carcinoma of the gallbladder: a unique approach to surgical palliation. Ann Res Hosp 2017;1:41.


