
Invasive and chronic fungal lung infections
Background
Fungi increasingly contribute to respiratory symptoms, causing a variety of clinical phenotypes such as invasive fungal disease (IFD) in the severely immunocompromised, chronic pulmonary disease in patients with local immune defects, allergic phenomena such as severe asthma with fungal sensitisation (SAFS) and allergic bronchopulmonary aspergillosis (ABPA). Treatment can be difficult and the antifungals currently in use can have significant side effect profiles. As such they can prove problematic for the general physician. Herein we review two clinical syndromes of invasive fungal lung infection and chronic fungal lung infection with readers directed to a recent excellent review regarding the allergic disorders (1).
Fungi are eukaryotic organisms, with a true nucleus and organelles. Their cell membranes contain ergosterol and are encased by a rigid cell wall composed of chitin, β-glucans, and mannans. They obtain nutrients by secreting hydrolytic enzymes that digest complex polymers, absorbing the resulting products. Fungi do not form complex organs, and exist either as single cells or a chain of cells (hyphae). Moulds are multicellular fungi that exist in the vegetative state as a mass of branching hyphae (mycelium), which have rigid cell walls, containing septae, and grow at the apex. Yeasts are single cells that propagate by budding, which may result in individual daughter cells, or a chain of attached cells. Some species can change their growth form during tissue invasion in humans, these are termed dimorphic. Fungi reproduce by sporulation, often asexual replicas of the parent cell (conidia), but others are capable of sexual reproduction, for example with the production of fruiting bodies such as mushrooms.
Fungal conidia are part of the airborne flora (2). Some conidia are the perfect size to be inhaled (e.g., Aspergillus species at 2–3 µm) and penetrate to terminal bronchi and alveoli (3), their ubiquitous nature (they are present in soils, water, dust, and in particular in decaying vegetation) ensure that we all breathe in some fungal spores on a daily basis. If not dealt with, they germinate into hyphae and secrete proteolytic enzymes which cause tissue damage, allowing tissue invasion. This results in parenchymal lung damage, angioinvasion, thrombosis, infarction and haematogenous dissemination. However they rarely cause disease, as unless the local immune system is deficient in some way, conidia are dealt with rapidly.
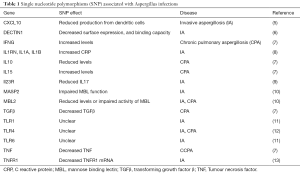
Initial barriers to disease are the mucociliary barrier, which involves physical clearing by cilia moving the mucous layer (4) and soluble proteins within the mucus with immune properties such as defensins, lysozyme, and secretory IgA. Once entering the alveolus, host defense is largely dependent on the presence and effectiveness of alveolar macrophages. These immune sentinels express cell surface pattern recognitions receptors (see Table 1), some of which recognize and bind fungal pathogen associated molecular patterns (PAMP). Dectin-1, a C-type lectin, binds β-glucan in the fungal cell wall, and induces phagocytosis as well as macrophage activation and consequent chemokine and inflammatory cytokine release. Toll like receptor (TLR) signalling molecules (TLR2, 4, 9) recognize a variety of fungal PAMPs such as zymosan, phospholipomannan, and fungal DNA.
Full table
The chemokine and inflammatory response induces the migration of neutrophils to the local alveolar environment. This vastly increases the phagocytic capacity of the local immune system, allowing fungal uptake and killing mediated by reactive oxygen species. Neutrophils are also capable of creating neutrophil extracellular traps that allow the killing of hyphae too large to engulf. The adaptive immune response also plays a role in fungal clearance. A dominant Th1 response, resulting in IFNγ secretion and opsonizing antibodies, increases phagocyte activity, and promotes fungal clearance. Conversely, a Th2 response promotes fungal allergic reactions, and Th17 mediated mucosal immunity is important in protection against mucocutaneous candidiasis.
IFD
Immunocompromised patients are at high risk of developing IFD, particularly patients with haematological malignancies, after haematopoietic stem cell transplants, and recipients of solid organ transplants. Up to 30% of patients not on antifungal prophylaxis develop a fever of unknown origin (FUO) when neutropenic, however the majority will have bacterial infections. Symptoms are non-specific and include dyspnoea, cough, pleuritic chest pain, and haemoptysis. Aspergillus species (most commonly A. fumigatus, but also A. flavus, A. terreus, A. nidulans and A. niger) account for the majority of pulmonary IFD (14), though other species such as Mucorales, and Fusarium have become more common (15). Pneumocystis jirovecii also causes respiratory infections in the immunocompromised but does not respond to antifungals, and is not considered further here. The endemic mycoses (Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, Paracoccidioides brasiliensis etc.) cause infections in the immunocompetent in defined geographical areas, and are not considered further here.
The ‘European Organization for Research and Treatment of Cancer’ (EORTC) has set out international consensus criteria for IFD (16) (Table 2). While these criteria were initially developed to guide inclusion into trials they provide a useful starting point to guide treatment with antifungals.

Full table
While microbiological culture is ideal, the sensitivity of microscopy and culture from bronchoalveolar lavage fluid (BALF) is no higher than 50% (17,18). Culture has the added value of allowing in vitro drug susceptibility testing, particularly with the increase in incidence of azole resistant Aspergillus isolates, though breakpoints are as yet not standardised. Notably the case fatality of patients with resistant Aspergillus is higher than non-resistant isolates (19).
Fungal cell wall components such as galactomannan (GM) and β-d-glucan (BDG) are now widely used to increase diagnostic sensitivity. GM is found in Aspergillus cell walls, so is particularly useful in the diagnosis of invasive aspergillosis (IA), with a serum GM of optical density (OD) <0.5 having a sensitivity of 41-78% and specificity of 60–95% (20), giving a 95% negative predictive value for IA (21,22). A single sample OD ≥1 or 2 separate serum samples OD ≥0.5 is considered positive by the FDA (https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM420236.pdf). OD is measured at 450nm, with OD expressed a ratio of sample compared to a ‘cut off’ control, with negative and positive controls establishing a valid test. Semi-synthetic β-lactams can cause false positive results up to 5 days after discontinuing them, as can cotton swabs, severe mucositis, severe gastrointestinal graft versus host disease, IgG myeloma and flavoured frozen desserts containing sodium gluconate. The use of mould active antifungals can cause false negative results (23), particularly in the context of surveillance (24). BALF levels of GM have good predictive value, both in haematological (21,25) and non-haematological patients (26), with a sensitivity of 87% and specificity of 89% using a cut off of ≥0.5, though many use a single sample (2 aliquots) cut off of ≥1 as positive (20). The data for serum GM in non-haematological patients is less good, with better results in BALF. A BALF GM of ≥0.5 was associated with >90% specificity in solid organ transplants, and specificity of 100% with a cut off of ≥1. Similarly in critical care patients, BAL GM ≥1 gives sensitivity of 100% and specificity of 89% (20).
BDG is expressed in most fungal cell walls (excluding Mucorales and Cryptococci), and can be used as a serum marker for both yeasts and moulds, though false positive results can be seen in with some Gram positive organisms, and the concomitant use of immunoglobulins or some dialysis membranes (27). This gives it an overall sensitivity of 77% and specificity of 85% (28) with a cut off of 80 pg/mL (intermediate 60–79 pg/mL). Aspergillus polymerase chain reaction (PCR) appears promising with high sensitivity in blood and BALF (29-31) but lack of standardization across laboratories has precluded its incorporation into recent IDSA diagnostic guidelines. A lateral flow device has been developed that gives point of care information on the presence of fungal cell wall constituents in respiratory samples, with sensitivity and specificity approaching that of PCR, with reported BALF sensitivities of 80-10% and specificity of 81–95% (20,32,33).
While conventional chest X-ray has low sensitivity for diagnosing IFD, the presence of nodules, ground glass halos, cavities and air crescent signs (34,35) can be seen in IA. Early cross sectional CT has been associated with earlier diagnosis (36) and is now standard of care when investigating unresolving fever in the immunocompromised patient.
As untreated pulmonary IFD has a high mortality rate [86%, (37,38)], there has been a move towards antifungal prophylaxis and early treatment of possible IFD. However, as antifungal drugs are costly and can cause significant toxic adverse events, there has been a drive towards stratifying patients using radiology, GM, and PCR prior to treatment with antifungals (39,40). The addition of biomarkers in the decision to commence antifungals significantly reduced antifungal use in a ‘real world’ Australian study (41). A recent meta-analysis comparing empirical antifungal therapy with diagnostic test-guided pre-emptive antifungal therapy, showed that a pre-emptive approach significantly reduced the use of antifungals (42) without affecting mortality. A cost analysis showed equipoise between the two groups, though slightly favouring the pre-emptive approach. This varied due to the cost of the drug used in the studies reviewed. However, as mortality rates are high, empirical antifungals are recommended in at risk patients who are persistently febrile despite broad-spectrum antibiotics whilst investigations are pending. There is no evidence of superior outcome if treatment was commenced on the day of fever, rather than after 4 days of broad spectrum antibiotics (43). This is because bacterial infections remain the commonest cause of fever in the neutropenic patient.
First line treatment of neutropenic patients with IA is with intravenous voriconazole (21) or liposomal Amphotericin B (44-46). A recent trial showed that isavuconazole was non-inferior to voriconazole with a marginally better adverse event profile (47). Posaconazole has not been trialled for primary IA, but salvage studies suggests it is effective for refractive IA (48). There is evidence that monitoring of therapeutic drug levels can help ensure therapeutic doses of azoles in blood (49), and improve outcome (48,50,51). The echinocandins have been approved for second line use, as they reduce mortality compared to placebo, though the mortality rates appear to be higher that with voriconazole (albeit without head to head trials) (52-54).
Combination therapy with two antifungals has been used for salvage therapy (55-57) though there are varied results from using combination therapy as first line therapy: with additional anidulafungin improving mortality rates (though not quite reaching statistical significance) compared to voriconazole alone (58), but the addition of caspofungin having no beneficial effect over voriconazole in another (59). Arguments have been advanced to use dual therapy upfront in the sickest patients. While there are theoretical reasons that azoles and polyenes may have antagonistic effects, this has not been borne out in clinical trials (60). Although there are little data on the treatment of breakthrough IA (i.e., with the use of prophylactic posaconazole), if therapeutic levels had been achieved, it seems sensible to recommend a class switch, usually to liposomal amphotericin.
Often treatment is blind, and rarely with information such as antifungal susceptibility patterns. GM correlates with fungal burden, and has been used to monitor response to treatment, and decay in levels are associated with successful outcome (61-63), with a >35% reduction in serum GM after 1 week associated with successful clinical outcome. Re-imaging after commencing treatments should not take place before 2 weeks, as there can be a temporary increase in the size of pulmonary lesions, in particular with neutrophil reconstitution (35). Usually antifungals are carried on for 6 to 12 weeks depending on clinical response, as many of the therapeutic trials have used 12 weeks survival as an endpoint, though there are little available data to support this duration of treatment.
Fusariosis, due to the Fusarium genera (most commonly F. solani and F. oxysporum) is second to IA as a cause of invasive mould disease and associated with prolonged neutropenia. In vitro testing suggests reduced susceptibility to the current range of antifungals, often seen in the context of breakthrough of patients on echinocandins or azoles (64-66). Compared to other IFD, there is an increase in rates of fungaemia and serum GM levels >0.5 have a sensitivity of up to 83% and specificity of 67% (67,68). Currently voriconazole, posaconazole and liposomal amphotericin are thought to be effective in fusariosis, though described survival rates at 12 weeks are only 33% to 42% (69,70) .
Mucormycosis, invasive mucormycete infection [commonly Rhizopus and Lichtheimia genera (71)], is difficult to differentiate from IA on clinical, radiological, and microbiological grounds and causes approximately 1.5% of IFD (72). Notably they have little GM in their cell wall, rendering this test ineffective. However the reverse halo sign is noted in early disease and targeted 18s PCR can be used to speed up diagnosis. They are constitutionally resistant to voriconazole, and treatment requires liposomal amphotericin, posaconazole or isavuconazole (+/− echinocandin). Mortality remains high (38–43% at 12 weeks) despite treatment (73,74). They have become responsible for healthcare associated outbreaks (75) with haematological malignancy and diabetes mellitus being particular risk factors.
Chronic pulmonary aspergillosis (CPA)
CPA is estimated to have a worldwide prevalence of 2.2 million people (76-78) and presents with non-specific symptoms such as cough, haemoptysis, fever, dyspnoea and weight loss. In particular it develops as a complication of lung damage due to tuberculosis, chronic obstructive pulmonary disease (COPD), ABPA, and sarcoid (79,80).
A 20 year retrospective analysis of 387 patients referred to a national centre showed a 5 year survival of 62%, with 98% patients on antifungal therapy. Factors that were associated with increased mortality included: coexisting non-tuberculous mycobacteria (NTM) infection, COPD, increased age, low albumin, low activity on St George’s Respiratory Questionnaire (SGRQ), increased MRC dyspnoea scores, and the presence of aspergillomas (especially bilateral) (81). A subset of these patients had fungal susceptibility information; patients with fully sensitive isolates had a 10-year survival of 68% in comparison to 46% in patients with any degree of azole resistance. Patients with pleural disease, cavitary disease and bilateral disease also had poorer outcomes compared to patients with nodules and intraluminal cavities, evident after 2 years.
Recent ERS guidelines have stratified the diagnosis of CPA (82), though an unbiased cluster analysis failed to separate patients by clinical criteria, suggesting while it can be useful to categorize patients, in reality there is a spectrum of disease (83). The diagnosis of chronic cavitary pulmonary aspergillosis (CCPA) requires the progression (enlargement and coalescence) of pleural or lung cavities with or without mycetomas or pleural thickening over ≥3 months with associated symptoms and either positive Aspergillus IgG serology or positive culture. Chronic fibrosing pulmonary aspergillosis (CFPA) presents as extensive fibrotic lung destruction with or without cavities and mycetomas, often this is the end result of untreated CCPA. Important differential diagnoses to exclude are mycobacterial infection, endemic mycoses (histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis) if there is an appropriate exposure history, and malignancy. The setting of NTM and CPA co-infection is not uncommon (84) and can be difficult to treat as rifampicin induction of cytochrome p450 enzymes can result in reduced azole levels (85).
Risk of subacute invasive aspergillosis (SAIA), previously chronic necrotising pulmonary aspergillosis, where symptoms and radiology progress over 1 to 3 months (86), is increased in patients with malnutrition, alcoholism, diabetes mellitus, prolonged steroid exposure, COPD, connective tissue disease, and HIV (87-89). Differential diagnoses include malignancy, vasculitides, and pulmonary infarctions. CPA can also present as aspergillus nodule and solitary aspergilloma, usually requiring surveillance rather than treatment in these contexts.
Aspergillus IgG is highly specific for CPA diagnosis in the context of progressive radiology and symptoms, with one small study reporting 100% positive predictive value (90). Two assays are commonly used to measure precipitating antibodies; the Platelia and Immunocap assays are similar at cut off values of >10 AU/mL and >40 mg/L respectively when discriminating between CPA and ABPA or other diseases (91), though the Immunocap appears to have a greater working range. The Platelia test had previously been shown to have a sensitivity of 90.2% to 93.8% and specificity of 99.5–100% when compared to healthy controls (though this reduced to 54–60% in comparison to patients with Aspergillus colonisation) (92), similarly the Immunocap has 98% sensitivity and 84% specificity with a cut off of 50 mg/L in a Japanese study of proven CPA (93). False negatives can appear in the context of hypogammaglobulinaemia, selective inability to produce A. fumigatus IgG (associated with functional deficiencies in pneumococcal and Haemophilus antibody), and non-aspergillus infections; e.g., with endemic mycoses or Scedosporium species. While there is little direct data, there is a suggestion that IgG titres reduce with successful treatment (94).
The value of serology and PCR is less well established in the context of CPA in comparison to IFA. A recent study evaluated BALF of CPA patients (95). They found that GM with a cut off of 0.5 ODI had a sensitivity of 78% and specificity of 90%, BDG (using Fungitec G test) with a cut off of 100 ODI, a sensitivity of 78% and specificity of 73%, and Aspergillus PCR sensitivity of 67–87% and specificity or 67–84%. If GM and BDG were used in combination, sensitivity was 67%, specificity 98%, positive predictive value 90% and negative predictive value 93%. Overall it appears that there is an inverse correlation between serum GM levels and Aspergillus IgG state when comparing aspergilloma, CPA, and IA (96).
CT is an important modality in diagnosing CPA syndromes, and repeat CT 6 months after commencing treatment has been shown to correlate with outcomes (97). Pleural and cavity wall thickness reduced with treatment, and resolution of mycetoma was also associated with clinical improvement, whereas changes in cavity volume, pericavitary infiltrates and consolidation were not.
The treatment of CPA is often long-term and can sometimes be poorly tolerated. Often the patients are frail or have significant comorbidities that may shorten their lifespan. Success rates are relatively poor with treatment goals often being symptom control rather than cure. Treatment is for a minimum of 6 months and often lifelong (98). A combination of symptom review, CT and serology can be used to guide treatment.
Oral voriconazole has a 53% success rate in treating SAIA and 14% success in CCPA (99), as determined by ≥50% radiological resolution and presumed mycological eradication. This was associated with subjective improvement in clinical symptoms assessed by visual analogue scale and reduction in serological response. This compares with historic studies with itraconazole considered stabilization of disease a success and reported approximately 20–30% radiological improvement (100,101), though it is clearly superior to supportive care (102). Posaconazole has a response rate of 61% at 6 months, and 46% at a year, where non-progression was considered a response (94). Retrospective analysis of SGRQ determined clinical symptoms suggests that posaconazole is superior to voriconazole which is in turn superior to itraconazole, though this must be viewed with caution without randomised control trial (RCT) head to head trials (103). It should be noted that many of these studies have heterogeneous participants and patients with SAIA have better treatment responses than those with CCPA, with only recent studies separating the two entities.
An RCT comparing voriconazole with micafungin indicated similar efficacy with micafungin having fewer adverse events (104). A further RCT show no difference in clinical response to CPA between caspofungin and micafungin, suggesting that this is little difference in the echinocandins (105). Additionally, case series have shown caspofungin can be effective in the scenario of progressive symptoms despite oral azole or with azole intolerance (106).
Liposomal amphotericin has been used as both induction therapy, for recalcitrant disease and in the setting or azole resistance. A case review indicated 74% of treated patients had clinical improvement (composite of MRC dyspnoea score and weight gain) after 6 months (107). A subset of these patients had recurrent courses of treatment with continuing symptom and serological improvement. Decline in renal function was widespread but only required cessation of therapy in 15% of cases, though this increased with repeated dosing.
Worryingly azole resistance, likely due to environmental fungicide use (108) has begun to emerge and has been associated with treatment failure (109-111), though it is unclear whether this can be reduced by ensuring appropriate therapeutic levels. Consensus expert opinion suggests the use of intermittent or continuous liposomal amphotericin or micafungin in the setting of pan azole resistance (112), and indeed where local resistance rates are >10% to begin treatment with liposomal amphotericin or with a voriconazole—echinocandin combination.
Azoles work by inhibiting fungal cytochrome P450, and can affect other drugs which are metabolized by human cytochrome p450 pathways, such as carbamazepine, so caution must be taken to take a full drug history and consider interactions before prescribing. Common azole adverse effects include hepatotoxicity, nausea, liver dysfunction, phototoxicity and visual disturbances. Measuring trough levels can be useful to minimise these side effects as well as ensuring therapeutic doses are being used. Polyenes (i.e., amphotericin) extract ergosterol from fungal cell membrane and the major side effect is renal failure, particularly when prescribed with other nephrotoxins. The echinocandins inhibit glucan and so cell wall synthesis. They are largely well tolerated with temporary infusion related fever and flushing being the commonest adverse event.
Conclusions
Fungal lung infection incidence and prevalence has increased over the past few decades, probably as a result of increased numbers of patients who are immunocompromised e.g., after transplant or on long term immunosuppressive agents such as high dose steroids. This has led to a cohort of patients with a spectrum of immune deficiencies that allow fungi to cause disease ranging from invasive infection with haematogenous spread to chronic indolent respiratory infection. These conditions can be difficult to diagnose with confidence and treatments can have significant toxicities particularly with prolonged consumption. It is important to have fungal pathogens in the differential of infective symptoms in the immunocompromised host and involve physicians familiar with treating them early during their course of treatment.
Acknowledgements
J Periselneris’s salary is supported by funding provided by Astellas, and D Armstrong-James has additionally received funding from Gilead.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73. [Crossref] [PubMed]
- Fischer G, Dott W. Relevance of airborne fungi and their secondary metabolites for environmental, occupational and indoor hygiene. Arch Microbiol 2003;179:75-82. [Crossref] [PubMed]
- Lacey ME, West JS. The Air Spora – A Manual for Catching and Identifying Airborne Biological Particles. Dordrecht, the Netherlands: Springer-Verlag Gmbh, 2006.
- Balloy V, Chignard M. The innate immune response to Aspergillus fumigatus. Microbes Infect 2009;11:919-27. [Crossref] [PubMed]
- Mezger M, Steffens M, Beyer M, et al. Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells. Blood 2008;111:534-6. [Crossref] [PubMed]
- Plantinga TS, van der Velden WJ, Ferwerda B, et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis 2009;49:724-32. [Crossref] [PubMed]
- Sambatakou H, Pravica V, Hutchinson IV, et al. Cytokine profiling of pulmonary aspergillosis. Int J Immunogenet 2006;33:297-302. [Crossref] [PubMed]
- Sainz J, Pérez E, Gómez-Lopera S, et al. IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol 2008;28:473-85. [Crossref] [PubMed]
- Carvalho A, Cunha C, Di Ianni M, et al. Prognostic significance of genetic variants in the IL-23/Th17 pathway for the outcome of T cell-depleted allogeneic stem cell transplantation. Bone Marrow Transplant 2010;45:1645-52. [Crossref] [PubMed]
- Granell M, Urbano-Ispizua A, Suarez B, et al. Mannan-binding lectin pathway deficiencies and invasive fungal infections following allogeneic stem cell transplantation. Exp Hematol 2006;34:1435-41. [Crossref] [PubMed]
- Kesh S, Mensah NY, Peterlongo P, et al. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann N Y Acad Sci 2005;1062:95-103. [Crossref] [PubMed]
- Bochud PY, Chien JW, Marr KA, et al. Toll-like Receptor 4 Polymorphisms and Aspergillosis in Stem-Cell Transplantation. N Engl J Med 2008;359:1766-77. [Crossref] [PubMed]
- Sainz J, Salas-Alvarado I, López-Fernández E, et al. TNFR1 mRNA expression level and TNFR1 gene polymorphisms are predictive markers for susceptibility to develop invasive pulmonary aspergillosis. Int J Immunopathol Pharmacol 2010;23:423-36. [Crossref] [PubMed]
- Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006;91:1068-75. [PubMed]
- Lamoth F, Chung S, Damonti L, et al. Changing Epidemiology of Invasive Mold infections in Patients Receiveing Azole Prophylaxis. Clin Infect Dis 2017;64:1619-21. [Crossref] [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Arendrup MC, Bille J, Dannaoui E, Ruhnke M, et al. ECIL-3 classical diagnostic procedures for the diagnosis of invasive fungal diseases in patients with leukaemia. Bone Marrow Transplant 2012;47:1030-45. [Crossref] [PubMed]
- Maschmeyer G, Carratalà J, Buchheidt D, et al. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients (allogeneic SCT excluded): updated guidelines of the Infectious Diseases Society of Hematology and Medical Oncology (DGHO). Ann Oncol 2015;26:21-33. [Crossref] [PubMed]
- van der Linden JW, Snelders E, Kampinga GA, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg Infect Dis 2011;17:1846-54. [Crossref] [PubMed]
- Miceli MH, Maertens J. Role of Non-Culture-Based Tests, with an Emphasis on Galactomannan Testing for the Diagnosis of Invasive Aspergillosis. Semin Respir Crit Care Med 2015;36:650-61. [Crossref] [PubMed]
- Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002;347:408-15. [Crossref] [PubMed]
- Maertens JA, Klont R, Masson C, et al. Optimization of the Cutoff Value for the Aspergillus Double-Sandwich Enzyme Immunoassay. Clin Infect Dis 2007;44:1329-36. [Crossref] [PubMed]
- Marr KA. Primary antifungal prophylaxis in hematopoietic stem cell transplant recipients: clinical implications of recent studies. Curr Opin Infect Dis 2008;21:409-14. [Crossref] [PubMed]
- Vena A, Bouza E, Álvarez-Uría A, et al. The misleading effect of serum galactomannan testing in high-risk haematology patients receiving prophylaxis with micafungin. Clin Microbiol Infect 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Maertens J, Van Eldere J, Verhaegen J, et al. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis 2002;186:1297-306. [Crossref] [PubMed]
- Fortún J, Martin-Davila P, Comez Garcia de la Pedrosa E, et al. Galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive aspergillosis in non-hematological patients. J Infect 2016;72:738-44. [Crossref] [PubMed]
- Marchetti O, Lamoth F, Mikulska M, et al. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant 2012;47:846-54. [Crossref] [PubMed]
- Karageorgopoulos DE, Vouloumanou EK, Ntziora F, et al. β-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 2011;52:750-70. [Crossref] [PubMed]
- Arvanitis M, Ziakas PD, Zacharioudakis IM, et al. PCR in diagnosis of invasive Aspergillosis: A Meta-Analysis of diagnostic performance. J Clin Microbiol 2014;52:3731-42. [Crossref] [PubMed]
- Heng SC, Morrissey O, Chen SC, et al. Utility of bronchoalveolar lavage fluid galactomannan alone or in combination with PCR for the diagnosis of invasive aspergillosis in adult hematology patients: A systematic review and meta-analysis. Crit Rev Microbiol 2015;41:124-34. [Crossref] [PubMed]
- Buchheidt D, Hummel M, Schleiermacher D, et al. Prospective clinical evaluation of a LightCycler-mediated polymerase chain reactoin assay, a nested PCR assay and a galactomannan enzyme-linked immunosorbent assay for detection of invasive aspergillosis in neutropenic cancer patients and haematological s. Br J Haematol 2004;125:196-202. [Crossref] [PubMed]
- Hoenigl M, Prattes J, Spiess B, et al. Performance of galactomannan, beta-d-glucan, aspergillus lateral-flow device, conventional culture, and pcr tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol 2014;52:2039-45. [Crossref] [PubMed]
- White PL, Parr C, Thornton C, et al. Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol 2013;51:1510-6. [Crossref] [PubMed]
- Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 2007;44:373-9. [Crossref] [PubMed]
- Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol 2001;19:253-9. [Crossref] [PubMed]
- Hauggaard A, Ellis M, Ekelund L. Early chest radiography and CT in the diagnosis, management and outcome of invasive pulmonary aspergillosis. Acta radiol 2002;43:292-8. [Crossref] [PubMed]
- Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis 1996;23:608-15. [Crossref] [PubMed]
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis Case-Fatality Rate: Systematic Review of the Literature. Clin Infect Dis 2001;32:358-66. [Crossref] [PubMed]
- Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis 2005;41:1242-50. [Crossref] [PubMed]
- Cordonnier C, Pautas C, Maury S, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis 2009;48:1042-51. [Crossref] [PubMed]
- Morrissey CO, Chen SCA, Sorrell TC, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: A randomised controlled trial. Lancet Infect Dis 2013;13:519-28. [Crossref] [PubMed]
- Fung M, Kim J, Marty FM, et al. Meta-analysis and cost comparison of empirical versus pre-emptive antifungal strategies in hematologic malignancy patients with high-risk febrile neutropenia. PLoS One 2015;10:e0140930. [Crossref] [PubMed]
- Maschmeyer G, Heinz WJ, Hertenstein B, et al. Immediate versus deferred empirical antifungal (IDEA) therapy in high-risk patients with febrile neutropenia: A randomized, double-blind, placebo-controlled, multicenter study. Eur J Clin Microbiol Infect Dis 2013;32:679-89. [Crossref] [PubMed]
- Cornely OA, Maertens J, Bresnik M, et al. Liposomal Amphotericin B as Initial Therapy for Invasive Mold Infection: A Randomized Trial Comparing a High-Loading Dose Regimen with Standard Dosing (AmBiLoad Trial). Clin Infect Dis 2007;44:1289-97. [Crossref] [PubMed]
- Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3—2009 Update. Bone Marrow Transplant 2011;46:709-18. [Crossref] [PubMed]
- Mousset S, Buchheidt D, Heinz W, et al. Treatment of invasive fungal infections in cancer patients-updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 2014;93:13-32. [Crossref] [PubMed]
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016;387:760-9. [Crossref] [PubMed]
- Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 2007;44:2-12. [Crossref] [PubMed]
- Guinea J, Escribano P, Marcos-Zambrano LJ, et al. Therapeutic drug monitoring of voriconazole helps to decrease the percentage of patients with off-target trough serum levels. Med Mycol 2016;54:353-60. [Crossref] [PubMed]
- Smith J, Safdar N, Knasinski V, et al. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother 2006;50:1570-2. [Crossref] [PubMed]
- Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis 2002;34:563-71. [Crossref] [PubMed]
- Maertens J, Raad I, Petrikkos G, et al. Efficacy and Safety of Caspofungin for Treatment of Invasive Aspergillosis in Patients Refractory to or Intolerant of Conventional Antifungal Therapy. Clin Infect Dis 2004;39:1563-71. [Crossref] [PubMed]
- Denning DW, Marr KA, Lau WM, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect 2006;53:337-49. [Crossref] [PubMed]
- Herbrecht R, Maertens J, Baila L, et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 2010;45:1227-33. [Crossref] [PubMed]
- Panackal AA, Parisini E, Proschan M. Salvage combination antifungal therapy for acute invasive aspergillosis may improve outcomes: a systematic review and meta-analysis. Int J Infect Dis 2014;28:80-94. [Crossref] [PubMed]
- Martín-Peña A, Aguilar-Guisado M, Espigado I, et al. Antifungal combination therapy for invasive aspergillosis. Clin Infect Dis 2014;59:1437-45. [Crossref] [PubMed]
- Caillot D, Thiébaut A, Herbrecht R, et al. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: A randomized pilot study (combistrat trial). Cancer 2007;110:2740-6. [Crossref] [PubMed]
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis a randomized trial. Ann Intern Med 2015;162:81-9. [Crossref] [PubMed]
- Raad II, Zakhem A, El , Helou G, El , et al. Clinical experience of the use of voriconazole, caspofungin or the combination in primary and salvage therapy of invasive aspergillosis in haematological malignancies. Int J Antimicrob Agents 2015;45:283-8. [Crossref] [PubMed]
- Candoni A, Caira M, Cesaro S, et al. Multicentre surveillance study on feasibility, safety and efficacy of antifungal combination therapy for proven or probable invasive fungal diseases in haematological patients: The SEIFEM real-life combo study. Mycoses 2014;57:342-50. [PubMed]
- Maertens J, Buvé K, Theunissen K, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer 2009;115:355-62. [Crossref] [PubMed]
- Fisher CE, Stevens AM, Leisenring W, et al. The serum galactomannan index predicts mortality in hematopoietic stem cell transplant recipients with invasive aspergillosis. Clin Infect Dis 2013;57:1001-4. [Crossref] [PubMed]
- Chai LY, Kullberg BJ, Johnson EM, et al. Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J Clin Microbiol 2012;50:2330-6. [Crossref] [PubMed]
- Chan TS, Gill H, Hwang YY, et al. Breakthrough invasive fungal diseases during echinocandin treatment in high-risk hospitalized hematologic patients. Ann Hematol 2014;93:493-8. [Crossref] [PubMed]
- Bose P, Parekh HD, Holter JL, et al. Disseminated fusariosis occurring in two patients despite posaconazole prophylaxis. J Clin Microbiol 2011;49:1674-5. [Crossref] [PubMed]
- Cudillo L, Girmenia C, Santilli S, et al. Breakthrough fusariosis in a patients with acute lymphoblastic leukemia receiving voriconazole prophylaxis. Clin Infect Dis 2005;40:1212-3. [Crossref] [PubMed]
- Mikulska M, Furfaro E, Del Bono V, et al. Galactomannan testing might be useful for early diagnosis of fusariosis. Diagn Microbiol Infect Dis 2012;72:367-9. [Crossref] [PubMed]
- Nucci M, Carlesse F, Cappellano P, et al. Earlier diagnosis of invasive fusariosis with Aspergillus serum galactomannan testing. PLoS One 2014;9:e87784. [Crossref] [PubMed]
- Litvinov N, da Silva MTN, van der Heijden IM, et al. An outbreak of invasive fusariosis in a children’s cancer hospital. Clin Microbiol Infect 2015;21:268.e1-268.e7. [Crossref] [PubMed]
- Pang KA, Godet C, Fekkar A, et al. Breakthrough invasive mould infections in patients treated with caspofungin. J Infect 2012;64:424-9. [Crossref] [PubMed]
- Garcia-Hermoso D, Alanio A, Cabaret O, et al. High diversity of non-sporulating moulds in respiratory specimens of immunocompromised patients: Should all the species be reported when diagnosing invasive aspergillosis? Mycoses 2015;58:557-64. [Crossref] [PubMed]
- Bitar D, Lortholary O, Strat Y, et al. Population-based analysis of invasive fungal infections. Emerg Infect Dis 2014;20:1149-55. [PubMed]
- Lanternier F, Poiree S, Elie C, et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother 2015;70:3116-23. [Crossref] [PubMed]
- Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016;16:828-37. [Crossref] [PubMed]
- Davoudi S, Graviss LS, Kontoyiannis DP. Healthcare-associated outbreaks due to Mucorales and other uncommon fungi. Eur J Clin Invest 2015;45:767-73. [Crossref] [PubMed]
- Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J 2013;41:621-6. [Crossref] [PubMed]
- Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 2013;51:361-70. [Crossref] [PubMed]
- Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ 2011;89:864-72. [Crossref] [PubMed]
- Koyama K, Ohshima N, Suzuki J, et al. Evaluation of clinical characteristics and prognosis of chronic pulmonary aspergillosis depending on the underlying lung diseases: Emphysema vs prior tuberculosis. J Infect Chemother 2015;21:795-801. [Crossref] [PubMed]
- Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J 2011;37:865-72. [Crossref] [PubMed]
- Lowes D, Al-Shair K, Newton PJ, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J 2017;49:1601062. [Crossref] [PubMed]
- Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016;47:45-68. [Crossref] [PubMed]
- Godet C, Laurent F, Beraud G, et al. Phenotyping chronic pulmonary aspergillosis by cluster analysis. Eur Respir J 2015;46:1509-12. [Crossref] [PubMed]
- Takeda K, Imamura Y, Takazono T, et al. The risk factors for developing of chronic pulmonary aspergillosis in nontuberculous mycobacteria patients and clinical characteristics and outcomes in chronic pulmonary aspergillosis patients coinfected with nontuberculous mycobacteria. Med Mycol 2016;54:120-7. [Crossref] [PubMed]
- Moon SM, Park HY, Jeong BH, et al. Effect of rifampin and rifabutin on serum itraconazole levels in patients with chronic pulmonary aspergillosis and coexisting nontuberculous mycobacterial infection. Antimicrob Agents Chemother 2015;59:663-5. [Crossref] [PubMed]
- Binder RE, Faling LJ, Pugatch RD, et al. Chronic necrotizing pulmonary aspergillosis: a discrete clinical entity. Medicine (Baltimore) 1982;61:109-24. [Crossref] [PubMed]
- Denning DW, Follansbee SE, Scolaro M, et al. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med 1991;324:654-62. [Crossref] [PubMed]
- Kim SY, Lee KS, Han J, et al. Semiinvasive pulmonary aspergillosis: CT and pathologic findings in six patients. AJR Am J Roentgenol 2000;174:795-8. [Crossref] [PubMed]
- Wessolossky M, Welch VL, Sen A, et al. Invasive aspergillus infections in hospitalized patients with chronic lung disease. Infect Drug Resist 2013;6:33-9. [PubMed]
- Uffredi ML, Mangiapan G, Cadranel J, et al. Significance of Aspergillus fumigatus isolation from respiratory specimens of nongranulocytopenic patients. Eur J Clin Microbiol Infect Dis 2003;22:457-62. [Crossref] [PubMed]
- Baxter CG, Denning DW, Jones AM, et al. Performance of two Aspergillus IgG EIA assays compared with the precipitin test in chronic and allergic aspergillosis. Clin Microbiol Infect 2013;19:E197-204. [Crossref] [PubMed]
- Guitard J, Sendid B, Thorez S, et al. Evaluation of a recombinant antigen-based enzyme immunoassay for the diagnosis of noninvasive aspergillosis. J Clin Microbiol 2012;50:762-5. [Crossref] [PubMed]
- Fujiuchi S, Fujita Y, Suzuki H, et al. Evaluation of a serology quantitative assay for diagnosing chronic pulmonary aspergillosis. J Clin Microbiol 2016;54:1496-9. [Crossref] [PubMed]
- Felton TW, Baxter C, Moore CB, et al. Efficacy and Safety of Posaconazole for Chronic Pulmonary Aspergillosis. Clin Infect Dis 2010;51:1383-91. [Crossref] [PubMed]
- Urabe N, Sakamoto S, Sano G, et al. Usefulness of two Aspergillus PCR assays and Aspergillus galactomannan and β-D-glucan testing of bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. J Clin Microbiol 2017;55:1738-46. [Crossref] [PubMed]
- Chan JF, Lau SK, Wong SC, et al. A 10-year study reveals clinical and laboratory evidence for the 'semi-invasive' properties of chronic pulmonary aspergillosis. Emerg Microbes Infect 2016;5:e37. [Crossref] [PubMed]
- Godet C, Laurent F, Bergeron A, et al. CT Imaging Assessment of Response to Treatment in Chronic Pulmonary Aspergillosis. Chest 2016;150:139-47. [Crossref] [PubMed]
- Koyama K, Ohshima N, Suzuki J, et al. Recurrence of chronic pulmonary aspergillosis after discontinuation of maintenance treatment by antifungal triazoles. J Infect Chemother 2014;20:375-9. [Crossref] [PubMed]
- Cadranel J, Philippe B, Hennequin C, et al. Voriconazole for chronic pulmonary aspergillosis: a prospective multicenter trial. Eur J Clin Microbiol Infect Dis 2012;31:3231-9. [Crossref] [PubMed]
- Dupont B. Itraconazole therapy in aspergillosis: study in 49 patients. J Am Acad Dermatol 1990;23:607-14. [Crossref] [PubMed]
- De Beule K, De Doncker P, Cauwenbergh G, et al. The treatment of aspergillosis and aspergilloma with itraconazole, clinical results of an open international study (1982-1987). Mycoses 1988;31:476-85. [Crossref] [PubMed]
- Agarwal R, Vishwanath G, Aggarwal AN, et al. Itraconazole in chronic cavitary pulmonary aspergillosis: A randomised controlled trial and systematic review of literature. Mycoses 2013;56:559-70. [Crossref] [PubMed]
- Al-Shair K, Atherton GT, Harris C, et al. Long-term antifungal treatment improves health status in patients with chronic pulmonary aspergillosis: A longitudinal analysis. Clin Infect Dis 2013;57:828-35. [Crossref] [PubMed]
- Kohno S, Izumikawa K, Ogawa K, et al. Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: A multicenter trial in Japan. J Infect 2010;61:410-8. [Crossref] [PubMed]
- Kohno S, Izumikawa K, Yoshida M, et al. A double-blind comparative study of the safety and efficacy of caspofungin versus micafungin in the treatment of candidiasis and aspergillosis. Eur J Clin Microbiol Infect Dis 2013;32:387-97. [Crossref] [PubMed]
- Keir GJ, Garfield B, Hansell DM, et al. Cyclical caspofungin for chronic pulmonary aspergillosis in sarcoidosis. Thorax 2014;69:287-8. [Crossref] [PubMed]
- Newton PJ, Harris C, Morris J, et al. Impact of liposomal amphotericin B therapy on chronic pulmonary aspergillosis. J Infect 2016;73:485-95. [Crossref] [PubMed]
- Chowdhary A, Kathuria S, Xu J, et al. Emergence of azole-resistant aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 2013;9:e1003633. [Crossref] [PubMed]
- Verweij PE, Mellado E, Melchers WJ. Multiple-Triazole-Resistant Aspergillosis. N Engl J Med 2007;356:1481-3. [Crossref] [PubMed]
- Howard SJ, Cerar D, Anderson MJ, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 2009;15:1068-76. [Crossref] [PubMed]
- Snelders E, Van Der Lee HA, Kuijpers J, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 2008;5:e219. [Crossref] [PubMed]
- Verweij PE, Ananda-Rajah M, Andes D, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 2015;21-22:30-40. [Crossref] [PubMed]
Cite this article as: Periselneris J, Armstrong-James D. Invasive and chronic fungal lung infections. Ann Res Hosp 2017;1:42.


