
HIV and respiratory illness in the antiretroviral therapy era
Introduction
Since the early reports in the 1980’s of Pneumocystis jirovecii pneumonia (PCP) as a common AIDS-defining illness, HIV infection has had an enduring relationship with respiratory disease. Without effective antiretroviral therapy (ART), people living with HIV (PLWH) are at greatly increased risk of pulmonary infections (1). As well as PCP, bacterial pneumonia and tuberculosis (TB) are both common. ART can suppress HIV replication and enable immune reconstitution such that the incidence of pulmonary infection falls significantly (2). It is uncertain whether the risk of respiratory infection falls completely to that of the HIV uninfected. In settings with good access to ART, this results in PLWH living considerably longer. With continued HIV transmission events and subsequent new infections, there is an expanding population who are now starting to age. Being HIV infected does not protect from the comorbidities associated with ageing, and may even promote them. As a result, we are now seeing a change in the pattern and tempo of respiratory illnesses that occur in PLWH. In this article, we review the bi-directional interaction between chronic HIV infection and respiratory illness.
Respiratory symptoms and HIV
Respiratory symptoms are common in PLWH and appear to be more frequent than in HIV negative populations. A systematic review and meta-analysis of studies reporting these in PLWH compared to HIV negative groups found a significantly higher prevalence in the former. The difference narrowed in populations with access to ART, such that in resource rich populations with access to ART the pooled odds ratio (OR) for reported breathlessness was 1.39 (95% CI: 1.11–1.73) (3). Figure 1 demonstrates the collated data from a number of such studies that have included information on self-reported breathlessness using the MRC score and compared PLWH with HIV negative controls.
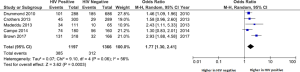
It should be noted, however, that studies evaluating respiratory symptoms in PLWH often cannot adjust for important confounding factors such as tobacco smoking (which is more common in HIV positive populations), and have often included a higher proportion of individuals who are not virologically suppressed despite using ART. Therefore, data from earlier reports may not be representative of a contemporary HIV positive population in many parts of the world.
A recent study from London of 197 HIV positive and 93 HIV negative participants specifically evaluated the prevalence of respiratory symptoms with the St George’s Respiratory Questionnaire (SGRQ) and Medical Research Council (MRC) dyspnoea scale. It found a greater frequency of respiratory symptoms in PLWH, with a median SGRQ of 12 (IQR 6–25) vs. 6 (IQR 2–14) in controls, P<0.001 (4). As the minimum clinically important difference between groups is usually regarded as four (5), this suggests that the PLWH had more reported symptoms. In line with this, 47% of the HIV positive group had MRC scores ≥2 (breathless going up an incline) compared to 24% in the HIV negative controls (P=0.001). After adjustment for confounding variables such as age, gender, smoking, body mass index (BMI) and depression, this difference persisted with the adjusted OR of an MRC score of ≥2 being 2.45 (95% CI: 1.15–5.20, P=0.02).
Although relatively few studies have measured respiratory symptoms in PLWH and compared these to matched HIV negative controls, current evidence suggests that symptoms such as cough and breathlessness are more common in HIV. There are likely to be multiple contributing causes including; higher rates of smoking tobacco and other substances, and increased non-communicable cardiovascular and respiratory illness. Despite attempts to control for this, it cannot be discounted that PLWH have a lower threshold to report symptoms, and so appear to have a greater symptom burden. The evidence for this is limited though factors such as higher depression scores in PLWH may contribute (4).
Infectious pulmonary disease in contemporary HIV populations
High rates of HIV suppression in the current HIV infected populations with access to ART have led to significant reductions in the morbidity and mortality associated with respiratory infection. However, a number of studies have concluded that the risk is still greater than in controls.
Data from the US Veterans care service reported by Crothers et al. (6) showed that rates of bacterial pneumonia, PCP and TB were still significantly greater in PLWH (28, 9.9 and 4.5/1,000 person years respectively) than in matched HIV negative controls (5.8, 0.02 and 0.6/1,000 person years). However, only 65% of this population were using ART, and the median CD4 count was 264 cells/µL, which would be regarded as at least moderately impaired immunity; and some way below the immune reconstitution that is generally achieved by increased ART coverage with good medication adherence.
Further data from the Women’s Interagency HIV study (WIHS) (a cohort developed in 1994) (7) and the Multicenter AIDS Cohort Study (MACS) (a cohort developed in 1984) (8) again demonstrated a higher risk of bacterial pneumonia in PLWH, although this has fallen significantly compared to the pre-ART era (9). For example, the MACS cohort reported a current adjusted OR for bacterial pneumonia in PLWH compared to controls of 4.14, whereas pre-ART this was 21.8. Of interest, the WIHS study showed a higher adjusted OR of 9.55 for bacterial pneumonia in HIV positive women with access to ART compared to matched controls. It is uncertain if this is due to innate biological factors, or reflects a common theme of worse access to care for women.
In contrast, data from the EuroSIDA collaboration of 18,000 patients in 36 predominantly European countries from 2006–2011 found a significantly lower incidence of bacterial pneumonia in the HIV positive population of 5.36/1,000 person years (10). The mean CD4 count in this population was 457 cells/µL and there were very low rates of severe bacterial infections including pneumonia in the subgroup with CD4 counts >500 cells/µL (generally recognised as a normal CD4 count). Although there was no HIV negative group in the study to allow direct comparison, other workers have estimated the incidence of bacterial pneumonia in the general population to lie between 5–11/1,000 person years (11-13).
Viral infections are a common cause of acute respiratory illness in all populations, and PLWH may be more susceptible to such infections, or more likely to develop severe disease compared to those without HIV. Clinical guidelines in the USA, UK and Europe therefore recommend annual immunisation against Influenza for all PLWH (14). There is however somewhat conflicting evidence as to whether PLWH are more likely to suffer severe viral infection. Cohen et al. (15) demonstrated an increased mortality in acute influenza infections in PLWH compared to HIV negative patients in South African and American populations. However, this excess mortality was reduced in the USA once ART was available. Subsequent studies during the H1N1 pandemic have shown no increase in deaths in HIV positive individuals (16).
There are little data directly comparing HIV suppressed PLWH using ART to HIV negative controls. It is likely that this will be available in the coming years, and will enable us to determine the current impact and significance of respiratory infection in a contemporary HIV population.
Figure 2 demonstrates a CT scan and a case vignette describing a number of different respiratory pathologies in a patient with advanced HIV and variable ART adherence.
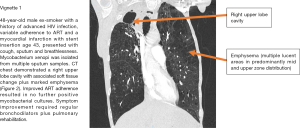
Non-communicable respiratory disease
Asthma
It is unclear whether PLWH are more or less likely to have asthma. In a cross-sectional analysis of 223 HIV infected participants, Gingo et al. reported a physician diagnosis of asthma in 20.6% (17). However, in the Veterans Ageing Cohort Study (VACS) (6) the prevalence of asthma was considerably lower (and in fact less than in an HIV negative control group: 2.0% vs. 2.4% respectively, P<0.001), whilst the incidence of new diagnoses of asthma was the same in the two populations. It is likely that the difference in the two US-based studies reflects the populations under test (e.g., risk for HIV, use of ART, age) and also the methods used to classify a patient as having asthma. For example, compared to the definition used by Gingo, the VACS study relied on an asthma ICD-9 coding having been recorded once as an inpatient or twice as an outpatient. There are little data regarding the effect of HIV status on asthma control.
An asthma study investigating a US Veteran’s Administration population of almost 26,000 (containing <1%, n=166, PLWH) reported a significant increase in all-cause mortality in HIV patients with asthma aged 18–45 (OR 3.64, 95% CI: 1.34–9.87) (18). Whilst this may have resulted from immunocompromise due to HIV (the study was performed when ART was not widely used), there was also a significantly increased incident rate ratio (IRR) for asthma-related hospitalisations in PLWH aged <65 (age 18–45, IRR 2.13 and age 45–65, IRR 1.91), suggesting that PLWH had more episodes of asthma resulting in admission to hospital (which would imply that these events were more severe). However, the small number of PLWH in the overall study argues for more focused work in this area.
Several studies after the advent of ART have shown that asthma is more common in children with vertically acquired HIV (19-21). Data from the National Institute of Health (NIH) Women and Infants transmission study (WITS) (22) demonstrated an increased cumulative incidence at age 13.5 years of asthma medication usage (as a proxy measure of asthma diagnosis) of 33.5% in HIV positive children on ART compared to 11.5% in HIV positive children not on ART. It has been suggested therefore, that the immune reconstitution associated with ART results in asthma symptoms, though the evidence for this is weak.
Chronic obstructive pulmonary disease
COPD has the potential to become a major comorbidity in PLWH. This may in part be a consequence of a higher prevalence of tobacco and recreational drug inhalation in many HIV positive populations, though also reflects the rising median age of most HIV cohorts using ART, and possibly the impact of chronic HIV infection itself. Crothers et al. (23). reported that in the VAC study, after adjusting for age, race, smoking intensity, injection drug use and alcohol misuse, there was still an increased odds of having a diagnosis of COPD in the HIV positive group compared to HIV negative controls [OR of ICD-9 coded diagnosis of COPD 1.47 (95% CI: 1.01–2.13) and for a self-reported diagnosis of COPD 1.58 (95% CI: 1.14–2.18)]. Further data from a larger sample of this cohort (33,000 HIV positive and over 66,000 HIV negative individuals) demonstrated a higher incidence of COPD in the PLWH (20.3 vs. 17.5/1,000 person years, P<0.001). The incidence was significantly greater in people with higher HIV load and lower in those who were taking ART at baseline, indicating that disease control may have an influence on the diagnosis and progression of COPD. Attia et al. found that HIV infection was an independent risk factor for radiological evidence of emphysema (defined as over 10% involvement on CT chest scans) in analyses adjusted for smoking rate (OR 2.24, 95% CI: 1.12–4.48) (24).
In the ALIVE cohort (25) of current and former injection drug users in Baltimore (in which approximately 30% of subjects are HIV positive), despite the HIV positive group having a lower baseline FEV1 and FVC, prospectively measured spirometry fell at the same rate over 3 years in HIV positive and HIV negative participants. However, when the HIV positive group was stratified by whether or not HIV suppression was achieved on ART, and also blood CD4 count above and below 100 cells/µL, there was a faster decline in spirometry in those with markers of worse immunity.
Although this implies that poorly controlled HIV may be driving a fall in lung function, the Strategic Timing of Antiretroviral Treatment (START) trial (26) (which randomised 1,026 PLWH with CD4 counts above 500 cells/µL to immediate or deferred ART) evaluated the rate of spirometric change in a pulmonary sub-study and found no significant difference between the groups in FEV1 decline over a two year follow up. Additionally, this appeared to be the same for smokers and non-smokers. It should be noted that the populations within the ALIVE and START studies are different, with the former having evidence of more advanced immunosuppression prior to study entry (25% had a blood CD4 count <200 cells/µL) compared to START subjects. This may in part explain the differences in results.
It is tempting to speculate that HIV infection itself may play a role in the development of COPD. Early data (27) suggesting that an accelerated form of emphysema in HIV infected smokers was dramatic and common appears to be less of an issue than originally thought. However milder CT-diagnosed emphysema has been reported in up to 25% of US adults. Unlike non-specific mild fibrotic changes (which in the same study were present in almost one third of participants), trace emphysema does not appear to be related to progressive immunocompromise (28).
A consistent finding from studies is that carbon monoxide diffusing capacity (DLCO) is reduced out of proportion to spirometry (29). This appears to be associated with symptoms such as breathlessness, cough or sputum (30). What underlies the lower DLCO is likely to be multifactorial, though it is of relevance that advanced HIV infection is associated with mucosal CD4 cell depletion and a CD8 alveolitis, and this appears to be reversed by ART (31). Whether drug therapy can slow or reverse clinically significant airway and alveolar abnormalities is less clear.
In summary, there are a number of studies recognising that COPD and lung function changes are common in HIV positive cohorts with no previous history of significant respiratory disease. There are less data investigating why this occurs, the precise contribution made by HIV infection and ART, compared to associated behavioural factors such as smoking, or whether it can be managed in part through immune modulation.
Pulmonary hypertension
The association between HIV and pulmonary arterial hypertension (PAH) was first noted in the late 1980s and studies from the early 1990s before the ready availability of ART reported a prevalence of PAH in PLWH as high as 0.5% (32). It is known that survival is worse in HIV-PAH patients not using ART compared to HIV negative patients with PAH (33), and PLWH without PAH (34), and that PAH is an independent risk factor for death in PLWH (35). The predominant symptom is rapidly progressive breathlessness and the median duration to diagnosis from onset of symptoms is 6 months. ART has significantly improved HIV associated mortality but the prevalence of HIV-PAH in some studies has remained static at 0.5% (36). This is still many times higher than the general population where the prevalence is recognised to be 10–52 cases per million (0.001% to 0.005%) (37). Opravil et al. (38) in the Swiss HIV cohort have shown a reduced incidence of HIV-PAH from 0.21% in 1995 to 0.03% in 2006 indicating that ART may have some effect. However, this significant reduction has not been reproduced elsewhere.
Figure 3 demonstrates a CT scan and case vignette of an HIV positive patient who developed lung cancer.
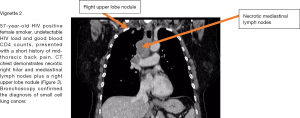
Lung cancer
Lung cancer is now the leading cause of cancer deaths in PLWH (39-41). This may be largely due to increased rates of smoking in this population, though HIV infection does appear to be an independent risk factor for lung cancer (42). PLWH appear to develop lung cancer at a younger age than HIV negative individuals (43).
Hessol et al. (44) studied participants from the WIHS and MACS cohorts. All but one incident lung cancer occurred in current or ex-smokers so further analysis was limited to those who had smoked ≥100 cigarettes prior to baseline assessment. When the two cohorts were combined, HIV infection was significantly associated with lung cancer incidence (IRR 2.64, 95% CI: 1.43–5.21). The unadjusted results from the MACS cohort also suggested an association between incident lung cancer diagnosis and lower blood CD4 counts, higher peak HIV load, and a prior AIDS diagnosis. This does not distinguish between a direct HIV effect and possible confounding variables. However, multivariable analysis of the combined data (1984–2011) indicated that older age, less education, a prior AIDS pneumonia diagnosis and ≥10 pack year smoking history were independently associated with a higher IRR of lung cancer.
Treatment for lung cancer in the HIV population lacks a strong evidence base as often HIV infection has been an exclusion criterion for clinical trials. A large population based study of 156,930 lung cancer patients (including 337 PLWH) reported to the Texas cancer registry from 1995–2009 (45) demonstrated that HIV infected patients with non-small cell lung cancer (NSCLC) were less likely to receive any cancer treatment than HIV uninfected individuals (60.3% vs. 77.5%, OR 0.39, 95% CI: 0.3–0.52) even after adjustment for year of diagnosis, age, sex, race, stage and histological subtype. HIV positive patients were also less likely to be surgically treated for local disease. Lung cancer mortality was higher in PLWH (Hazard ratio 1.34, 98% CI: 1.15–1.56) and HIV positive patients were more likely to present with disseminated disease. This study was limited by lack of data on CD4 count, use of ART, socioeconomic status, medical insurance performance status and comorbid illness, all of which may affect access to, and suitability for, cancer treatment. The full explanation for this disparity is likely to be multifactorial and may include lack of specific guidelines for HIV- related lung cancer, concern about drug interactions with ART and possibly a historical belief that HIV infection significantly reduces Performance Status. This serves to highlight the need for joined up specialist care, multiprofessional working, sustained healthcare worker and public education programmes.
As PLWH on ART now have a life expectancy similar to the general population and minimal impairment of Performance Status, it is likely that more data will become available on lung cancer treatment in this group.
Sleep disordered breathing
Fatigue and daytime somnolence are commonly reported symptoms in PLWH. There can be significant overlap between the description of these and breathlessness. There is conflicting evidence regarding the contribution sleep disordered breathing makes to these symptoms.
Cross-sectional data from VACS reported a prevalence of obstructive sleep apnoea (OSA) in HIV positive individuals of 3.9% compared to 12.4% in an HIV negative population (46). Patil et al. found an extremely high rate of sleep disordered breathing in males within MACS (47). This was present in PLWH using ART, those not on ART and the HIV negative group (71%, 74% and 87% respectively). The authors concluded that there may have been an element of reporting bias considering the high prevalence in all groups. Gingo et al., using data from MACS and WIHS, found that the prevalence of sleep apnoea was similar in HIV infected men and women when compared to the uninfected group (<5% for both groups) (48). When this data were adjusted for measures such as age, race, BMI and smoking status there was an increased prevalence of sleep apnoea in the PLWH in both cohorts compared to the HIV negative group with a prevalence ratio (PR) of 1.42 (95% CI: 1.1–1.84, P=0.01) in MACS and 2.10 (95% CI: 1.31–3.36, P=0.002) in WIHS. Of note, one of the major acknowledged risks for OSA, raised BMI, was no more prevalent in the HIV population. Other possible factors that might contribute to OSA include upper airway abnormalities due to tonsillar and adenoid hypertrophy, plus the adverse effects of ART and other medication that can cause myopathy and abnormal body fat redistribution (lipohypertrophy).
As with so many aspects of HIV related respiratory disorders, more work is required to better understand the relationship between HIV and sleep disordered breathing/OSA, fatigue and somnolence; and how specific therapy may improve these symptoms.
Improving the respiratory health of PLWH
The extent to which HIV is an independent risk factor for respiratory illness in individuals using effective ART is uncertain. There is a need for more high-quality studies with well-matched HIV negative controls in both adults and children. These should seek to answer specific questions about the “natural history” of respiratory illness in contemporary populations, and also test out potentially effective interventions that will reduce respiratory and associated morbidities. A good example of this is the need to ensure that there is maximal and ongoing, effective ART usage in all PLWH. This calls for improved access to healthcare in both resource rich and poor environments.
In many settings PLWH are at greater risk of respiratory ill health due to higher exposure to known drivers such as tobacco smoke. For example, in European and North American studies, PLWH are significantly more likely to smoke than the general population (49). They may also be less likely to quit (50). There is a need, therefore, to understand how services can be developed to help people who want to stop smoking. Unfortunately, not only is this often a low priority for HIV services, but also the current models of care provision may be unsuitable. For example, whilst PLWH smoking rates are apparently high, they are in fact similar to HIV negative matched controls, suggesting that the “message” needs to be directed “upstream” of HIV care if we are to achieve good quit rates (4).
As well as addressing the frequency of tobacco smoking and recreational drug use, respiratory health can be maintained by ensuring access to immunisations against vaccine-preventable respiratory illness such as Pneumococcal pneumonia and influenza. Both of which are recommended for PLWH (14,51), with evidence of reasonable uptake in the UK. (52) It is important, however, to ensure that the provision of these immunisations meets the needs of the HIV positive population and so maximises their impact (53).
Although the risk of TB is significantly reduced by the provision of ART (2), PLWH remain at greater risk of TB than the general population, in particular those who have migrated from high TB incidence settings. Current guidelines provide differing advice regarding who should be screened for latent TB infection and offered preventive treatment. Also, the changing demographics of TB in many, generally resource rich locations mean that current guideline recommendations soon may not be cost-effective (54). There is a need to improve the evidence base supporting preventative strategies for TB for PLWH. Improved diagnostic tests with a better ability to predict who is at risk of developing TB than the currently available immune-based tuberculin skin tests and TB interferon gamma release assays would significantly improve the benefit and cost-effectiveness of such testing.
Lung cancer screening in PLWH is potentially useful. The National Lung Screening Trial (55) in the USA enrolled 53,454 current or former smokers aged 55–74 (≥30 pack years’ history) without symptoms or signs of lung cancer, and unknown HIV status. It showed that annual low dose helical CT screening led to a relative reduction in mortality of lung cancer of 20% (95% CI: 6.8–26.7, P=0.004) compared to annual screening by chest radiograph. It is perhaps then intuitive that CT screening in HIV patients with higher risk of lung cancer might reduce mortality. A multicentre feasibility single CT study in France (56) of 442 HIV positive smokers (≥20 pack years) with median age 49.8 years and a nadir CD4 count ≤350 cells/µL found a positive CT result (defined as a significant nodule, adenopathy or endobronchial image) in 94 (21%). 15 went on to have further invasive procedures, without significant complication, and lung cancer was diagnosed in 10 (2.3%), of which 9 had an abnormal baseline scan. Six of these were diagnosed at early stages and eight patients were aged less than 55 years (and hence would not have been assessed if using the National Lung Screening Trial criteria). The number of screening tests to detect one cancer was 49, which compares favourably with general population screening. Further work is required to better understand the possible benefits of lung cancer screening, including which patients are at greatest risk for lung cancer and hence would benefit most from a screening programme, the frequency of repeat CT imaging in people with initially normal scans and what to do with the inevitably large number of non-specific pulmonary nodules identified in PLWH.
The bringing together of existing population-based respiratory health programmes such as smoking cessation, pulmonary rehabilitation, home oxygen, sleep and home ventilation services and lung cancer MDT management, with potentially stand-alone HIV care providers is an important component of long term, high quality HIV-related respiratory disease management and requires planning.
Conclusions
Improving and maintaining respiratory health is an important part of care for PLWH. The provision of ART has led to a change in the epidemiology of respiratory disease in this population with a reduction in acute opportunistic respiratory infections and a growing burden of chronic non-communicable respiratory disease. This presents challenges for the provision of HIV healthcare services, which now have to manage acute respiratory illnesses and also offer long term effective public health measures such as tobacco control, immunisations for vaccine-preventable infections, and the complexity of multiple non-AIDS comorbidities alongside HIV care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wallace JM, Hansen NI, Lavange L, et al. Respiratory disease trends in the Pulmonary Complications of HIV Infection Study cohort. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 1997;155:72-80. [Crossref] [PubMed]
- Gupta RK, Rice B, Brown AE, et al. Does antiretroviral therapy reduce HIV-associated tuberculosis incidence to background rates? A national observational cohort study from England, Wales, and Northern Ireland. Lancet HIV 2015;2:e243-51. [Crossref] [PubMed]
- Brown J, Roy A, Harris R, et al. Respiratory symptoms in people living with HIV and the effect of antiretroviral therapy: a systematic review and meta-analysis. Thorax 2017;72:355-66. [Crossref] [PubMed]
- Brown J, McGowan J, Chouial H, et al. Respiratory health status is impaired in UK HIV-positive adults with virologically suppressed HIV infection. HIV Med 2017;18:604-12. [Crossref] [PubMed]
- Jones PW, Quirk F, Baveystock C. The St George's respiratory questionnaire. Respiratory medicine 1991;85:25-31. [Crossref] [PubMed]
- Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011;183:388-95. [Crossref] [PubMed]
- Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology 1998;9:117-25. [Crossref] [PubMed]
- Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987;126:310-8. [Crossref] [PubMed]
- Gingo MR, Balasubramani GK, Kingsley L, et al. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS One 2013;8:e58812. [Crossref] [PubMed]
- Sogaard OS, Reekie J, Ristola M, et al. Severe bacterial non-aids infections in HIV-positive persons: incidence rates and risk factors. J Infect 2013;66:439-46. [Crossref] [PubMed]
- Woodhead MA, Macfarlane JT, McCracken JS, et al. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987;1:671-4. [Crossref] [PubMed]
- Jokinen C, Heiskanen L, Juvonen H, et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol 1993;137:977-88. [Crossref] [PubMed]
- Foy HM, Cooney MK, Allan I, et al. Rates of pneumonia during influenza epidemics in Seattle, 1964 to 1975. Jama 1979;241:253-8. [Crossref] [PubMed]
- Geretti AM, Brook G, Cameron C, et al. British HIV Association Guidelines on the Use of Vaccines in HIV-Positive Adults 2015. HIV Med 2016;17 Suppl 3:s2-s81. [Crossref] [PubMed]
- Cohen C, Simonsen L, Sample J, et al. Influenza-related mortality among adults aged 25-54 years with AIDS in South Africa and the United States of America. Clin Infect Dis 2012;55:996-1003. [Crossref] [PubMed]
- Sheth AN, Althoff KN, Brooks JT. Influenza susceptibility, severity, and shedding in HIV-infected adults: a review of the literature. Clin Infect Dis 2011;52:219-27. [Crossref] [PubMed]
- Gingo MR, Wenzel SE, Steele C, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol 2012;129:708-14.e8. [Crossref] [PubMed]
- Sumino K, O'Brian K, Bartle B, et al. Coexisting chronic conditions associated with mortality and morbidity in adult patients with asthma. J Asthma 2014;51:306-14. [Crossref] [PubMed]
- Siberry GK, Leister E, Jacobson DL, et al. Increased risk of asthma and atopic dermatitis in perinatally HIV-infected children and adolescents. Clin Immunol 2012;142:201-8. [Crossref] [PubMed]
- Foster SB, Paul ME, Kozinetz CA, et al. Prevalence of asthma in children and young adults with HIV infection. J Allergy Clin Immunol 2007;119:750-2. [Crossref] [PubMed]
- Gutin F, Butt A, Alame W, et al. Asthma in immune-competent children with human immunodeficiency virus. Ann Allergy Asthma Immunol 2009;102:438. [Crossref] [PubMed]
- Foster SB, McIntosh K, Thompson B, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol 2008;122:159-65. [Crossref] [PubMed]
- Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326-33. [Crossref] [PubMed]
- Attia EF, Akgun KM, Wongtrakool C, et al. Increased risk of radiographic emphysema in HIV is associated with elevated soluble CD14 and nadir CD4. Chest 2014;146:1543-53. [Crossref] [PubMed]
- Drummond MB, Merlo CA, Astemborski J, et al. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS 2013;27:1303-11. [Crossref] [PubMed]
- Kunisaki KM, Niewoehner DE, Collins G, et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med 2016;4:980-9. [Crossref] [PubMed]
- Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med 1992;116:124-8. [Crossref] [PubMed]
- Leader JK, Crothers K, Huang L, et al. Risk Factors Associated With Quantitative Evidence of Lung Emphysema and Fibrosis in an HIV-Infected Cohort. J Acquir Immune Defic Syndr 2016;71:420-7. [Crossref] [PubMed]
- Fitzpatrick ME, Gingo MR, Kessinger C, et al. HIV infection is associated with diffusing capacity impairment in women. J Acquir Immune Defic Syndr 2013;64:284-8. [Crossref] [PubMed]
- Crothers K, McGinnis K, Kleerup E, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr 2013;64:271-8. [Crossref] [PubMed]
- Popescu I, Drummond MB, Gama L, et al. Activation-induced Cell Death Drives Profound Lung CD4(+) T-Cell Depletion in HIV-associated Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2014;190:744-55. [Crossref] [PubMed]
- Speich R, Jenni R, Opravil M, et al. Primary pulmonary hypertension in HIV infection. Chest 1991;100:1268-71. [Crossref] [PubMed]
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation 1994;89:2722-7. [Crossref] [PubMed]
- Opravil M, Pechere M, Speich R, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 1997;155:990-5. [Crossref] [PubMed]
- Nunes H, Humbert M, Sitbon O, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2003;167:1433-9. [Crossref] [PubMed]
- Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008;177:108-13. [Crossref] [PubMed]
- Hoeper MM, Simon RGJ. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014;23:450-7. [Crossref] [PubMed]
- Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. Aids 2008;22 Suppl 3:S35-40. [Crossref] [PubMed]
- Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753-62. [Crossref] [PubMed]
- Winstone TA, Man SF, Hull M, et al. Epidemic of lung cancer in patients with HIV infection. Chest 2013;143:305-14. [Crossref] [PubMed]
- Morlat P, Roussillon C, Henard S, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. Aids 2014;28:1181-91. [Crossref] [PubMed]
- Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012;26:1017-25. [Crossref] [PubMed]
- Robbins HA, Engels EA, Pfeiffer RM, et al. Age at cancer diagnosis for blacks compared with whites in the United States. J Natl Cancer Inst 2015.107. [PubMed]
- Hessol NA, Martinez-Maza O, Levine AM, et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. Aids 2015;29:1183-93. [Crossref] [PubMed]
- Suneja G, Shiels MS, Melville SK, et al. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. Aids 2013;27:459-68. [Crossref] [PubMed]
- Kunisaki KM, Akgun KM, Fiellin DA, et al. Prevalence and correlates of obstructive sleep apnoea among patients with and without HIV infection. HIV Med 2015;16:105-13. [Crossref] [PubMed]
- Patil SP, Brown TT, Jacobson LP, et al. Sleep disordered breathing, fatigue, and sleepiness in HIV-infected and -uninfected men. PLoS One 2014;9:e99258. [Crossref] [PubMed]
- Gingo MR, Balasubramani GK, Rice TB, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med 2014;14:75. [Crossref] [PubMed]
- Miners A, Phillips A, Kreif N, et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. The Lancet HIV 2014;1:e32-e40. [Crossref] [PubMed]
- Goldberg D, Weber KM, Orsi J, et al. Smoking cessation among women with and at risk for HIV: are they quitting? J Gen Intern Med 2010;25:39-44. [Crossref] [PubMed]
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:309-18. [Crossref] [PubMed]
- Ellis J, Brown J, Smith C, et al. Influenza immunisation: knowledge and actions taken by UK HIV-positive adults. HIV Med 2016;17:397-9. [Crossref] [PubMed]
- Pickett E, Brown J, van Schalkwyk MCI, et al. Access to influenza immunisation services by HIV positive patients in the UK. Influenza Other Respir Viruses 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Capocci S, Smith C, Morris S, et al. Decreasing cost effectiveness of testing for latent TB in HIV in a low TB incidence area. Eur Respir J 2015;46:165-74. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Makinson A, Eymard-Duvernay S, Raffi F, et al. Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. Aids 2016;30:573-82. [Crossref] [PubMed]
Cite this article as: Jaffer A, Devani N, Brown J, Mandal S, Lipman M. HIV and respiratory illness in the antiretroviral therapy era. Ann Res Hosp 2017;1:43.


