
Protective role of Phoenix dactylifera fruit against ethanol-induced gastric ulcer in Wistar rats
Introduction
Peptic ulcers are pathological gastrointestinal lesions that occur in the stomach and duodenum as a result of imbalance between the defensive gastric mucosa and aggressive factors such as gastric acids and pepsin, free radicals, leukotrienes, ethanol, Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs (NSAIDs) etc. (1-3). Gastric ulcer is characterized by inflammation, hemorrhagic erosions, necrosis, infiltration of neutrophils, reduction of blood flow, reactive oxygen species (ROS) and oxidative stress (4,5). A major source of ROS in ethanol-induced gastric mucosal damage is the infiltration of neutrophils (2). Neutrophils adhere to the vascular endothelium and subsequently release oxygen-derived free radicals and proteolytic enzymes that are implicated in the pathogenesis of various forms of gastrointestinal ulceration including gastric mucosal damage associated with oral ethanol consumption or administration (6-8). Hydrogen receptor antagonist, proton pump inhibitors, antacids, histamine receptor antagonist are the commonly used antiulcer drugs (9,10). However, most of these drugs result in undesirable adverse side effects; hypergastrinemia and high rate of ulcer recurrence due to long term use (11-14). Therefore, there is a need for a natural and readily available antiulcer drug with little or no side effect. Phoenix dactylifera (P. dactylifera), commonly known as date palm, is a flowering plant species in the family Arecaceae, cultivated for its edible sweet fruit; the plant is widely cultivated and distributed in many tropical and subtropical regions worldwide (15). Studies have shown that date extract enhances antioxidant processes and prevent oxidative stress the antioxidant potential was attributed to the wide range of phenolic compounds, flavonoids, tannins and saponins and vitamin C (16,17). Date palm fruit extracts were reported to exhibit gastrointestinal protection against different ulcer inducing agents (18,19). It also possesses antioxidant, hepatoprotective, anti-ulcerative, anti-inflammatory, anti-proliferative, anti-mutagenic, antibacterial, antifungal and antiviral potentials (20,21). P. dactylifera extracts was proven to exhibit antagonist effects to oxidative stress induced by opioid (22). The aim of the present study was to evaluate the protective role of P. dactylifera extract against ethanol-induced gastric mucosal damage by evaluating the antioxidant defensive mechanism along with morphological and histopathological changes.
Methods
Plant material
P. dactylifera fruits were purchased from a local salesman in Maiduguri central market and were authenticated by a botanist in University of Maiduguri. The fruit were separated from the pit, washed, air dried and grounded with mortar and pestle. Twenty grams of the powdered fruit was soaked in 1,000 mL of distilled water for 24 hours, filtered with a filter paper and allowed to settle down, it was decanted and oven dried at 50 °C.
Animal Husbandry
Twenty healthy and pathogen free male Wistar Rats were purchased from the Department of Biochemistry Animal House, University of Maiduguri. They were fed with grower mash (Vital Feed, Jos, Nigeria) and water ad libitum. The rats were kept in the Department of Biochemistry animal House for 2 weeks to acclimatize with the animal house condition before the experiment. The research was approve by the Department of Human Anatomy, University of Maiduguri and was given the code number UM/HA/UGP16.17-004, it was conducted in accordance with the guidelines of University of Maiduguri Research and Ethical Committee, the ARRIVE guidelines by National Center for the Replacement Refinement and Reduction of Animals in Research (NC3Rs) and Helsinki Declaration as revised in 2013.
Experimental design
The rats were randomly divided into four groups and fasted for 18 hours. Rats in groups 1 and 2 were pretreated with 1 mL normal saline and gestid suspension (Ranbaxy Pharmaceuticals Inc., Princeton, NJ, USA) respectively while those in groups 3 and 4 were pretreated with aqueous extract of P. dactylifera fruit at 250 and 500 mg/kg respectively 30 minutes before administration of 1 mL of 80% ethanol. The LD 50 of P. dactylifera fruit was reported to be greater than 3,000 mg/kg (23) 17% of the LD 50 was chosen as the high dose. All the rats were sacrificed after 1 hour by ketamine injection and the stomachs were dissected out and cut opened, the stomach content were deposited in a beaker and 3–5 drops of pH meter was added to determine the pH. The stomachs were washed and examined using a hand lens for macroscopic lesions in the glandular part. The severity of macroscopic lesions formed were estimated using an ulcer index (UI) as reported by (24) using the following scale: 0 for normal mucosa; 1 for vascular congestions; 2 for one or two lesions; 3 for severe lesions; 4 for very severe lesions and 5 for mucosa full of lesions. The total and ulcerated area of each stomach was measured and ulcer indices (UIs), ulcer inhibition rates (UIRs) and total stomach area (TSA) were calculated using the formulae; UI = UA/TSA ×100 (24,25), UIR (%) = UI (control–pretreated)/UI control ×100. TSA = πr2 where, UA = ulcerated area, π =3.14 and r = diameter of stomach/2 (25,26).
Histopathology
The stomach of each rat was fixed in 10% formalin, and processed for light microscopic study; they were sectioned at 5 µm and stained with hematoxylin and eosin (H & E) (27).
Statistical analysis
One way analysis of variance (ANOVA) was used to compare the differences between the groups using Instat statistics 3.1 (Graphpad Software, San Diego, CA, USA). All the data were expressed as mean ± SEM and a P value less than 0.05 was considered as the significant level.
Results
There was significant decrease in the pH of rats pre-treatments with 250 mg/kg (5.70±0.83) and 500 mg/kg (5.50±0.67) aqueous extracts of P. dactylifera as compared with that of rats pre-treated with normal saline (7.90±0.60) and gestid suspension (8.50±0.27) at P<0.05 (Table 1). The pH of stomach content of rats pre-treated with normal saline and gestid suspension were alkaline while those of rats pre-treated with aqueous extract of P. dactylifera at 250 and 500 mg/kg were acidic.

Full table
From Table 2, the UI of rats pre-treatments with 250 mg/kg (6.68±2.83) and 500 mg/kg (9.40±3.00) of P. dactylifera as was significantly lower than those of rats pre-treated with normal saline (55.91±6.94) at P<0.05, there was dose dependent increase in the UI of rats pre-treatments with aqueous extracts of P. dactylifera at 250 mg/kg (6.68±2.83) and 500 mg/kg (9.40±3.00) respectively with significant decrease in UI of rats pre-treated with gestid suspension (4.80±3.06) as compared with rats pretreated with 250 and 500 mg/kg of P. dactylifera extract. There was a decrease in the UIR of rats pre-treated with 250 mg/kg (88.54±4.52) and 500 mg/kg (77.99±11.29) aqueous extracts of P. dactylifera as compared with the rats pre-treated with gestid suspension (92.49±4.67) at P<0.05. Rats pre-treated with aqueous extract of P. dactylifera at 250 mg/kg showed lower UI (6.68±2.83) than those of rats pre-treated with aqueous extract of P. dactylifera at 500 mg/kg (9.40±3.00) and normal saline (55.91±6.94), but has a little higher UI than rats pre-treated with gestid suspension (4.80±3.06), the UIR of rats pre-treated with aqueous extract of P. dactylifera at 250 mg/kg (88.54±4.52) is higher than those of rats pre-treated with the extract at 500 mg/kg (77.99±11.29) but lower than those of rats pre-treated with gestid suspension (92.49±4.67) (Table 2).
Full table
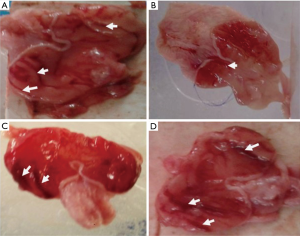
The gross anatomy of the stomach of rats pre-treated with normal saline showed mucosa full of lesions while those of rats pre-treated with gestid suspension showed few vascular congestions, the gross anatomy of the stomach of rats pre-treated with aqueous extract of P. dactylifera at 250 mg/kg showed few mild lesions while those of rats pre-treated with and aqueous extract of P. dactylifera at 500 mg/kg showed few mild lesions few severe lesions (Figure 1).
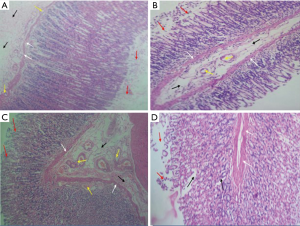
Photomicrograph of stomach of rats pre-treated with normal saline showed loss of luminal epithelium, distortion of submucosal layer and weakness of muscularis mucosa with encroaching blood vessels (Figure 2A). Photomicrograph of stomach of rats pre-treated with gestid suspension showed distortion of submucosal layer, normal muscularis mucosa, normal blood vessels within sub-mucosal layer and loss of luminal mucosal epithelium (Figure 2B). Photomicrograph of stomach of rats pre-treated with aqueous extract of P. dactylifera at 250 mg/kg showing distortion of submucosal layer, normal muscularis mucosa with some blood vessel within the mucosal layer and a little distortion of luminal mucosal layer (Figure 2C). Photomicrograph of stomach of rats pre-treated with aqueous extract of P. dactylifera at 500 mg/kg showing normal muscularis mucosa, distortion mucosal layer around the gastric pits (black arrows) and loss of luminal mucosal epithelium (Figure 2D).
Discussion
The slightly alkaline pH of stomach content of rats pre-treated with normal saline and gestid suspension showed that normal saline does not contain any substance that will require the secretion of gastric juice while protecting the gastric mucosa by either preventing gastric acid secretion or neutralizing the effect of the acid secreted by gastric glands. The acidic pH of stomach content of rats pre-treated with aqueous extract of P. dactylifera at 250 and 500 mg/kg showed that the extract does not protect the stomach mucosa by either preventing gastric acid secretion or neutralizing the gastric acid secreted by the stomach glands, the preventive mechanism might be due to the presence of flavonoids, tannins, saponins and vitamin c that serve as antioxidants to clear the oxygen-derived free radicals and proteolytic enzymes that are implicated in the parthenogenesis of ethanol induced gastric ulcers (6,7). Because of the high nutrient content of P. dactylifera extract (28,29), there is need for gastric secretion to create a favourable acidic pH that will initiate digestion.
The significant decrease in the UI of rats pre-treated with P. dactylifera extract at 250 and 500 mg/kg showed that the extract can protect the stomach mucosa from damage due to ethanol consumption, even though gestid suspension offers better protection as having lower UI as compared with that of P. dactylifera extract. P. dactylifera extract is most effective at 250 mg/kg and because it is a natural product with many medicinal properties (30), it is likely to present little or no side effects.
The high UIR of P. dactylifera extract showed that the extract is not only effective in reducing UI but is also effective in inhibiting the rate of ulcer formation and progression, making the extract better than some ulcer drugs that results in high rate of ulcer recurrence with long term use (11,12).
The extract of P. dactylifera at 250 mg/kg was shown to reduce gastric mucosal epithelial damage, distortion of submucosal layer and maintaining endothelial cell integrity, this might be due to the fact that P. dactylifera extract contains phenolic compounds and has antioxidant property (30) but at a higher dose of 500 mg/kg, the resulted mucosal damage might be due to the presence of saponin in the extract that was proven to create pores and exert a lytic action on some cell membranes leading to cell damage (31,32).
Conclusions
Aqueous extract of P. dactylifera was able to protect the stomach against ethanol-induced gastric ulcer by its antioxidant property of scavenging free radicals, reducing UI, increasing UIR, reducing gastric lesions, reducing gastric mucosal damage and maintaining the integrity of endothelial epithelium.
Acknowledgments
We wish to acknowledge the effort and contributions of Mustapha Ali, Freda Nathan Abwage, Johnson Adamu, Ladi Yahi Mingila, Mustapha Ahmed Bukar, Moloh Immaculate, Martha Orendu Attah and Helga Bedan Ishaya.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The research was conducted in accordance with the University of Maiduguri Research and Ethical Committee guide for the care and use of laboratory animals, the ARRIVE guidelines by the National Center for the Replacement Refinement and Reduction of Animals in Research and the provisions in accordance with the Helsinki Declaration as revised in 2013.
References
- Chen H, Liao H, Liu Y, et al. Protective effects of pogostone from Pogostemonis Herba against ethanol-induced gastric ulcer in rats. Fitoterapia 2015;100:110-7. [Crossref] [PubMed]
- El-Maraghy SA, Rizk SM, Shahin NN. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem Biol Interact 2015;229:26-35. [Crossref] [PubMed]
- Zeren S, Bayhan Z, Kocak FE, et al. Gastroprotective effects of sulforaphane and thymoquinone against acetylsalicylic acid induced gastric ulcer in rats. J Surg Res 2016;203:348-59. [Crossref] [PubMed]
- Viana AF, Fernandes HB, Silva FV, et al. Gastroprotective activity of Cenostigma macrophyllum Tul. var. acuminata Teles Freire leaves on experimental ulcer models. J Ethnopharmacol 2013;150:316-23. [Crossref] [PubMed]
- Adeniyi OS, Emikpe BO, Olaleye SB. Gastric mucosa re-epithelisation, oxidative stress and apoptosis during healing of acetic acid-induced ulceration in thyroxine treatment and thyroidectomy on rats. J Afr Ass Physiol Sci 2014;2:57-66.
- Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J 1996;10:731-40. [PubMed]
- Rocha NF, Oliveira GV, Araújo FY, et al. (-)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur J Pharm Sci 2011;44:455-61. [Crossref] [PubMed]
- Jiang M, Gao PF, Li HQ, et al. Ghrelin inhibition of ethanol-induced gastric epithelial cell apoptosis is mediated by miR-21. Int J Clin Exp Pathol 2015;8:4662-72. [PubMed]
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009;374:1449-61. [Crossref] [PubMed]
- Ribeiro AR, Diniz PB, Pinheiro MS, et al. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, ATP-sensitive K(+) channels, and gastric mucus secretion. Chem Biol Interact 2016;244:121-8. [Crossref] [PubMed]
- Saxena B, Singh S. Investigations on gastroprotective effect of citalopram, an antidepressant drug against stress and pyloric ligation induced ulcers. Pharmacol Rep 2011;63:1413-26. [Crossref] [PubMed]
- Abdelwahab SI. Protective mechanism of gallic acid and its novel derivative against ethanol-induced gastric ulcerogenesis: involvement of immunomodulation markers, Hsp70 and Bcl-2-associated X protein. Int Immunopharmacol 2013;16:296-305. [Crossref] [PubMed]
- Kangwan N, Park JM, Kim EH, et al. Quality of healing of gastric ulcers: natural products beyond acid suppression. World J Gastrointest Pathophysiol 2014;5:40-7. [Crossref] [PubMed]
- Xie M, Chen H, Nie S, et al. Gastroprotective effect of gamma-aminobutyric acid against ethanol-induced gastric mucosal injury. Chem Biol Interact 2017;272:125-34. [Crossref] [PubMed]
- Janick J. The origin of fruits, fruit growing and fruit breeding. Plant Breeding Rev 2005;25:255-320.
- Mansouri A, Embarek G, Kokkalou E, et al. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem 2005;89:411-20. [Crossref]
- Naskar S, Islam A, Mazumder UK, et al. In Vitro and In Vivo Antioxidant Potential of Hydromethanolic Extract of Phoenix dactylifera Fruits. J Sci Res 2010;2:144-57.
- Al-Qarawi AA, Ali BH, Al-Mougy SA, et al. Gastrointestinal transit in mice treated with various extracts of date (Phoenix dactylifera L.). Food Chem Toxicol 2003;41:37-9. [Crossref] [PubMed]
- Al-Qarawi AA, Abdel-Rahman H, Ali BH, et al. The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. J Ethnopharmacol 2005;98:313-7. [Crossref] [PubMed]
- Tariq N, Jenkins DJ, Vidgen E, et al. Effect of soluble and insoluble fiber diets on serum prostate specific antigen in men. J Urol 2000;163:114-8. [Crossref] [PubMed]
- Mallhi TH, Qadir MI, Ali M, et al. Review: Ajwa date (Phoenix dactylifera)- an emerging plant in pharmacological research. Pak J Pharm Sci 2014;27:607-16. [PubMed]
- Sani IH, Bakar NHA, Rohin MAK, et al. Phoenix dactylifera linn as a potential novel antioxidant in treating major opioid toxicity. J App Pharm Sci 2015;5:167-72. [Crossref]
- Akunna GG, Saalu CL, Ogunmodede OS, et al. Aqueous Extract of Date Fruit (Phoenix Dactylifera) Protects Testis against Atrazine-induced Toxicity in Rat. World J Life Sci Med Res 2012;2:100-8.
- Sabiu S, Garuba T, Sunmonu T, et al. Indomethacin-induced gastric ulceration in rats: Protective roles of Spondias mombin and Ficus exasperata. Toxicol Rep 2015;2:261-7. [Crossref] [PubMed]
- Mshelia HS, Karumi Y, Dibal NI. Therapeutic effect of Momordica balsamina leaf extract on ethanol-induced gastric ulcer in Wistar rats. Ann Res Hosp 2017;1:1-5. [Crossref]
- Boligon AA, De Freitas RB, De Brum TF, et al. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm Sin B 2014;4:358-67. [Crossref] [PubMed]
- Yam MF, Ang LF, Salman IM, et al. Orthosiphon stamineusleaf extract protects against ethanol-induced gastropathy in rats. J Med Food 2009;12:1089-97. [Crossref] [PubMed]
- Sadiq IS, Izuagie T, Shuaibu M, et al. The Nutritional Evaluation and Medicinal Value of Date Palm (Phoenix dactylifera). Int J Modern Chem 2013;4:147-54.
- Yasin BR, El-Fawal HA, Mousa SA. Date (Phoenix dactylifera) Polyphenolics and Other Bioactive Compounds: A Traditional Islamic Remedy's Potential in Prevention of Cell Damage, Cancer Therapeutics and Beyond. Int J Mol Sci 2015;16:30075-90. [Crossref] [PubMed]
- Al-Alawi RA, Al-Mashiqri JH, Al-Nadabi JSM, et al. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front Plant Sci 2017;8:845. [Crossref] [PubMed]
- El Izzi A, Benie T, Thieulant ML, et al. Stimulation of LH release from cultured pituitary cells by saponins of Petersianthus macrocarpus: a permeabilizing effect. Planta Med 1992;58:229-33. [Crossref] [PubMed]
- Francis G, Kerem Z, Makkar HP, et al. The biological action of saponins in animal systems: a review. Br J Nutr 2002;88:587-605. [Crossref] [PubMed]
Cite this article as: Musa MA, Dibal NI, Chiroma MS, Makena W. Protective role of Phoenix dactylifera fruit against ethanol-induced gastric ulcer in Wistar rats. Ann Res Hosp 2017;1:46.


