Sepsis and community-acquired pneumonia
Community-acquired pneumonia (CAP) is a common disease, and among infectious diseases it is the major cause of death (1,2). CAP may cause local and systemic inflammation, whether or not invasive disease is present (3,4). The systemic compromise is the consequence of a dysregulated host response and may lead to organ dysfunctions such as renal failure, neurologic compromise, and septic shock, and eventually to death (5).
Prompt, rapid diagnosis is essential in order to provide effective treatment and to improve outcomes. Recently, the definition of sepsis has changed, generating a great deal of discussion on the best methods for recognizing the condition and for assessing the risk of death (6).
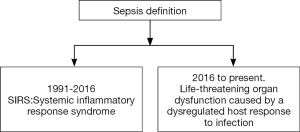
In 1991, sepsis was defined as a systemic response to infection (7). The concept was termed systemic inflammatory response syndrome (SIRS) and was defined as the presence of more than one of the following clinical manifestations: (I) body temperature above 38 °C or below 36 °C; (II) heart rate above 90 beats per minute; (III) tachypnea, manifested by a respiratory rate above 20 breaths per minute, or hyperventilation, indicated by a PaCO2 below 32 mmHg; and (IV) an alteration in the white blood cell count, i.e., above 12,000/cu mm or below 4,000/cu mm, or the presence of more than 10% immature neutrophils (“bands”). These parameters are the expression of physiologic changes caused by infection; SIRS frequently leads to organ dysfunction, though not in all cases.
In 2001 a committee (Sepsis 2) (8) revised the definition, but maintained the concept of SIRS. A list of non-specific signs that might be present in sepsis and in early organ dysfunction was added to help identify septic patients. The committee also added the concept of PIRO, the acronym of a system for staging sepsis which takes account the Predisposition (underlying factors such as premorbid illness, age or factors that reduced short-term survival), insult infection (pathogens and specific toxins such as LPS), response (systemic inflammatory response) and organ dysfunction (number of failing organs).
In 2004 (9), a campaign called Surviving Sepsis was launched, designed to reduce sepsis-related mortality by improving early and effective management of septic patients. Several updates have since been published (10-12).
In 2016, a new committee (Sepsis 3) revised the concept of sepsis after some studies had shown that SIRS did not accurately predict poor outcomes (13,14). This committee introduced a profound change in the concept of sepsis (Figure 1), defining it as life-threatening organ dysfunction caused by a dysregulated host response to infection. The concept of SIRS was eliminated, and for practical management, sepsis is defined by an increase in the Sequential (Sepsis-related) Organ Failure Assessment (SOFA) (15) score of two points or more, associated with an in-hospital mortality above 10%. In addition, for prompt, rapid identification in the emergency department or general ward, a new bedside clinical score was developed called Quick SOFA (qSOFA), which includes a respiratory rate of 22/min or greater, altered mentation, or systolic blood pressure of 100 mmHg or less. A score of two points or more indicates a high risk of poor outcome.
Prevalence of sepsis in CAP according to different definitions
CAP is the most common cause of sepsis in many of the series reported (16,17). Between 40–50% of patients with sepsis present respiratory sources of infection. CAP is also the leading cause of death among infectious diseases, and so these patients should be extensively evaluated for early recognition of poor outcomes.
In a recent analysis of two cohorts in Spain, 79% of patients presented sepsis according to the Sepsis 1–2 definition, and 62% according to the Sepsis 3 definition (18). In the PORT cohort, 82% of patients had two SIRS criteria; however, organ dysfunction was found in 48% of patients with CAP, and 5% developed septic shock. The SIRS criteria were not associated with an increased likelihood of progression to severe sepsis (odds ratios of 0.65 for two or more SIRS criteria and 0.89 for three or more) (13).
Score validation (are sepsis scores better than PSI and CURB65 for predicting 30-day mortality?)
Several studies carried out since Sepsis-3 have evaluated the best approach for early recognition of patients with sepsis and have validated the concept of qSOFA. Some of these studies were performed in patients with CAP.
Wang and colleagues (19) evaluated the use of qSOFA in the Emergency Department and observed that qSOFA showed similar results to SOFA or APACHE II, but was inferior to Mortality in Emergency Department Sepsis (MEDS).
SIRS has shown high sensitivity but low specificity, while qSOFA has shown low sensitivity (20). As noted by Williams and colleagues (21), this observation has limited the use of qSOFA. Interestingly, the presence of SIRS increases the risk of death, even in patients without organ dysfunction.
SOFA has proved to be a better predictive model than other scores when comparing patients admitted to ICU (22). However, it is difficult to calculate and so its use may delay early recognition.
Two large cohort studies from Europe and the US support the use of qSOFA for recognition of patients with high risk of poor outcomes instead of SIRS (23,24). In the study by Freund and colleagues, the highest areas under the curve receiving operating characteristic (AUROC) were for the qSOFA score (0.80; 95% CI, 0.74–0.85) and the SOFA score (0.77; 95% CI, 0.71–0.82) compared with 0.65 (95% CI, 0.59–0.70) for SIRS and 0.65 (95% CI, 0.59–0.70) for severe sepsis (P <0 .001, compared with qSOFA). The study of Donnelly and colleagues found that 1-year mortality after discharge was also higher for patients with elevated qSOFA scores [29.4 deaths (95% CI, 22.3–38.7) per 100 person-years] compared with those with elevated SOFA scores [22.6 deaths (19.2–26.6) per 100 person-years] or those who met SIRS criteria [14.7 deaths (12.5–17.2) per 100 person-years].
In a recent meta-analysis (25), the analysis of sensitivity for the diagnosis of sepsis comparing qSOFA and SIRS favored SIRS [1.32 (0.40–2.24), P<0.0001, I2=100%], but the analysis of the AUROC in six studies comparing qSOFA and SIRS favored qSOFA [0.03 (0.01–0.05), P=0.002, I2=48%] as a predictor of in-hospital mortality.
The study by Ranzani and colleagues (18), conducted in two cohorts of patients with CAP from Hospital Clinic in Barcelona and Hospital La Fe in Valencia, reported that 442 of 6,874 patients (6.4%) died in hospital. SIRS presented the worst discrimination (AUROC 0.579, 95% CI, 0.551–0.605), followed by qSOFA (AUROC 0.697, 95% CI, 0.671–0.722), CRB (AUROC 0.716, 95% CI, 0.690–0.741) and SOFA (AUROC 0.748, 95% CI, 0.721–0.774). Calibration plots were comparable among the scores, although overestimation was more pronounced for qSOFA and SOFA scores. Using the cut-off of two points, the sensitivity/specificity was 88/22% for SIRS, 50/82% for qSOFA, 39/87% for CRB and 97/23% for SOFA. The net benefit of the SIRS strategy was similar to that of the treat-all strategy, and was higher for SOFA and qSOFA. Interestingly, in this study the score proposed for management of CAP outperformed qSOFA and SOFA, CRB-65 was better than qSOFA as a bedside clinical score, and PSI was better than SOFA for predicting mortality. Chen and colleagues (26) evaluated the performance of qSOFA and compared it with CRB-65 in patients with pneumonia; both scores include neurologic status, respiratory rates and blood pressure, although they used different cut-off points. CRB-65 also included age, a known risk factor for mortality in pneumonia. The authors observed that qSOFA was better than CRB-65 for predicting mortality, even though one limitation of this study is the high mortality observed.
In view of these results, the assessment of risk in patients with CAP we propose that the scores developed for this condition, mainly CURB-65 (27) and PSI (28), and ATS/IDSA criteria (29) should continue to be used for site of care determinations. These scores have been extensively validated in CAP, include variables that are readily available, and are commonly used for CAP management (30).
Biomarkers in sepsis and CAP
Several markers can be used for diagnosis or prognosis in patients with sepsis and CAP.
C-reactive protein (CRP) is an acute phase reactant protein. Its levels increase during inflammation, and bacterial infections produce a rapid rise (4,31,32). Changes in the levels of CRP can be used to diagnose sepsis; however, they may be more useful for monitoring patient response (33). A fall in CRP levels indicates a good response to treatment. CRP may also be used to indicate adjunctive treatments, as discussed below (34).
Procalcitonin (PCT) is a marker of inflammation, and increases particularly in systemic bacterial infections (35). High levels of PCT indicate a high likelihood of systemic infection (36). PCT is also useful for guiding antibiotic prescription in lower airway respiratory infection and for supporting decisions to discontinue antibiotic therapy (37).
Lactate is a marker of tissue hypoperfusion, and is used as a marker of severe sepsis and septic shock. High lactate levels are associated with increased risk of organ failure and mortality (38). Levels above 4 mmol/L in the context of sepsis are considered as septic shock (39). Lactate levels can be used as a dynamic marker for fluid resuscitation and requirement of vasopressor therapy. Lactate should be used carefully in patients with renal and hepatic failure, given that its clearance depends on the kidney and the liver.
MR-proAdrenomedullin (MR-proADM) is the most stable fragment of the adrenomedullin precursor, and is used as a marker of sepsis. It has mainly been evaluated in CAP. Its prognostic value at admission is comparable with that of clinical scores such as PSI and CURB-65, and is independent of the etiology of CAP (40). It is also a marker for fluid sequestration, endothelial damage and heart failure, and can therefore be used dynamically during fluid resuscitation (41,42).
Influence of sepsis on outcomes of CAP
The presence of sepsis and organ dysfunction in patients with CAP is a risk factor for poor outcome (13), especially for patients who presented septic shock or required mechanical ventilation (43,44). The mortality rate rose to 33% in patients requiring mechanical ventilation and to 25% in patients with septic shock. Patients with organ dysfunction other than septic shock or respiratory failure also had higher mortality (45-47); however, the impact of these dysfunctions on outcomes was lower than that of septic shock or requirement of mechanical ventilation (44,48,49).
Patients may present organ dysfunction at admission, or develop it during hospitalization. Several studies showed that patients admitted to ICU after first passing through the general ward have worse outcomes and higher mortality (51 to 23% vs. 23 to 12%) (50-52). Many of these patients might benefit from early and intensive treatment.
Management of patients with sepsis and CAP
Early recognition and diagnosis allows prompt initiation of therapy (12). Sepsis and septic shock are medical emergencies and should be treated immediately. Resuscitation from sepsis hypoperfusion should be started with 30 ml/kg of IV crystalloid fluid within three hours (53-56). Hemodynamic assessment including cardiac functions should be performed dynamically, and additional fluid use should be guided by frequent reassessment of hemodynamic status, with the goal of maintaining a mean blood pressure above 65 mmHg. Lactate levels can also be used to evaluate sepsis-related hypoperfusion. Rivers’s protocol (57), also known as early goal-directed therapy, has been proposed for the management of sepsis primarily due to its good results in the trial. This protocol included a central venous pressure higher than 8 cmH2O, maintenance of mean blood pressure above 65 mmHg, and central venous oxygenation saturation higher than 70%. Despite the good results obtained by Rivers and cols, these results could not be reproduced in three subsequent RCTz (53-56). If hemodynamic stability is not achieved with fluid resuscitation, vasopressor therapy should be initiated. Norepinephrine is the first-choice vasopressor; vasopressin or epinephrine may be added, and dopamine is an alternative to norepinephrine. Dobutamine should be added in patients who show evidence of persistent hypoperfusion despite adequate fluid loading and the use of vasopressor agents.
Respiratory support should be added if necessary. Oxygen support via mask, high flow nasal cannula or mechanical ventilation is recommended.
Before starting antimicrobial treatments, microbiologic cultures should be performed, although these cultures must not delay the initiation of antimicrobial therapy. At least two sets of blood cultures, including aerobic and anaerobic cultures and when possible respiratory secretions, should be obtained. Isolation of the causal germ allows appropriate antibiotic de-escalation in a carefully managed antibiotic stewardship program (12,29). New molecular techniques will also allow rapid identification of the germ and resistance patterns (58); however, more validation studies are required.
Intravenous antibiotics should be administered as soon as possible, within an hour of recognition of sepsis. Any delay in their administration increases the risk of mortality, length of stay, complication rate and SOFA score (59,60).
Empirical antibiotic treatment should cover the main pathogens that cause pneumonia, including Streptococcus pneumoniae, atypical bacteria and Gram-negative bacilli. Guidelines suggest the use of a β-lactam plus a macrolide, β-lactam plus a fluoroquinolone or a fluoroquinolone alone as empirical treatment (29,61). In a Spanish multicenter study of more than 4,000 patients, adherence to guidelines and early antibiotic administration were protective factors against 30-day mortality in patients with sepsis and CAP (62). Interestingly, Amaro and colleagues (63) showed that patients who received antibiotics prior to hospital admission were less likely to present septic shock or require mechanical ventilation.
Macrolide combinations showed better outcomes in retrospective and observational studies than monotherapy with a β-lactam, especially in critically ill patients (64-67). Macrolides also have an immunomodulatory effect in addition to an antimicrobial effect.
In patients with severe CAP and high inflammatory response, corticosteroids may be added if the patient does not present contraindication or Influenza pneumonia. Corticosteroid use reduced treatment failure, a composite outcome that included early treatment failure (development of shock, need for invasive mechanical ventilation and death in the first 72 hours), and late treatment failure (persistence of respiratory failure, radiographic progression, shock, and need for mechanical ventilation or death among 72 to 120 hours after admission) (34). Corticosteroids have also shown reduced time to clinical stability and length of stay in several RCTs and reduced mortality in patients with severe CAP in meta-analyses, though some of the RCTs included had a high risk of bias (68).
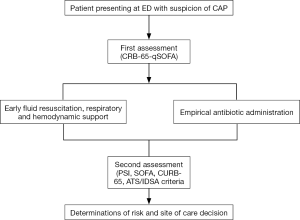
Other adjunctive therapies are under study for use in patients with severe pneumonia, such as enrichment immunoglobulin in patients with CAP requiring mechanical ventilation. Results of a phase II study showed benefits for patients with low levels of immunoglobulin or high values of CRP (69). An algorithm with initial management of patients with CAP and sepsis is showed in Figure 2.
Conclusions
Patients presenting with CAP and sepsis must be identified quickly and treated with effective antibiotic treatment within the first hour of ER admission. There is some controversy about the scores for risk assessment; however, CAP scores such as PSI and CURB-65 have been extensively validated and are suitable for use.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ramirez JA, Wiemken TL, Peyrani P, et al. Adults Hospitalized With Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin Infect Dis 2017;65:1806-12. [Crossref] [PubMed]
- Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 2015;386:1097-108. [Crossref] [PubMed]
- Ceccato A, Torres A, Cilloniz C, et al. Invasive Disease vs Urinary Antigen-Confirmed Pneumococcal Community-Acquired Pneumonia. Chest 2017;151:1311-9. [Crossref] [PubMed]
- Martínez R, Menéndez R, Reyes S, et al. Factors associated with inflammatory cytokine patterns in community-acquired pneumonia. Eur Respir J 2011;37:393-9. [Crossref] [PubMed]
- Menéndez R, Montull B, Reyes S, et al. Pneumonia presenting with organ dysfunctions: Causative microorganisms, host factors and outcome. J Infect 2016;73:419-26. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. [Crossref] [PubMed]
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73. Erratum in: Crit Care Med 2004;32:2169-70. Crit Care Med 2004;32:1448. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327. Erratum in: Crit Care Med 2008;36:1394-6. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486-552. [Crossref] [PubMed]
- Dremsizov T, Clermont G, Kellum JA, et al. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest 2006;129:968-78. [Crossref] [PubMed]
- Vincent JL, Opal SM, Marshall JC, et al. Sepsis definitions: time for change. Lancet 2013;381:774-5. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303-10. [Crossref] [PubMed]
- Alberti C, Brun-Buisson C, Chevret S, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med 2005;171:461-8. [Crossref] [PubMed]
- Ranzani OT, Prina E, Menéndez R, et al. New Sepsis Definition (Sepsis-3) and Community-acquired Pneumonia Mortality. A Validation and Clinical Decision-Making Study. Am J Respir Crit Care Med 2017;196:1287-97. [Crossref] [PubMed]
- Wang JY, Chen YX, Guo SB, et al. Predictive performance of quick Sepsis-related Organ Failure Assessment for mortality and ICU admission in patients with infection at the ED. Am J Emerg Med 2016;34:1788-93. [Crossref] [PubMed]
- Giamarellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al. Validation of the new Sepsis-3 definitions: proposal for improvement in early risk identification. Clin Microbiol Infect 2017;23:104-9. [Crossref] [PubMed]
- Williams JM, Greenslade JH, McKenzie JV, et al. Systemic Inflammatory Response Syndrome, Quick Sequential Organ Function Assessment, and Organ Dysfunction: Insights From a Prospective Database of ED Patients With Infection. Chest 2017;151:586-96. [Crossref] [PubMed]
- Raith EP, Udy AA, Bailey M, et al. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017;317:290-300. [Crossref] [PubMed]
- Freund Y, Lemachatti N, Krastinova E, et al. Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients With Suspected Infection Presenting to the Emergency Department. JAMA 2017;317:301-8. [Crossref] [PubMed]
- Donnelly JP, Safford MM, Shapiro NI, et al. Application of the Third International Consensus Definitions for Sepsis (Sepsis-3) Classification: a retrospective population-based cohort study. Lancet Infect Dis 2017;17:661-70. [Crossref] [PubMed]
- Serafim R, Gomes JA, Salluh J, et al. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome Criteria for the Diagnosis of Sepsis and Prediction of Mortality: A Systematic Review and Meta-Analysis. Chest 2018;153:646-55. [Crossref] [PubMed]
- Chen YX, Wang JY, Guo SB. Use of CRB-65 and quick Sepsis-related Organ Failure Assessment to predict site of care and mortality in pneumonia patients in the emergency department: a retrospective study. Crit Care 2016;20:167. [Crossref] [PubMed]
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377-82. [Crossref] [PubMed]
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243-50. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Ranzani OT, Taniguchi LU, Torres A. Severity scoring systems for pneumonia: current understanding and next steps. Curr Opin Pulm Med 2018;24:227-36. [PubMed]
- Póvoa P, Coelho L, Almeida E, et al. C-reactive protein as a marker of infection in critically ill patients. Clin Microbiol Infect 2005;11:101-8. [Crossref] [PubMed]
- Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax 2009;64:587-91. [Crossref] [PubMed]
- Póvoa P, Coelho L, Almeida E, et al. Pilot study evaluating C-reactive protein levels in the assessment of response to treatment of severe bloodstream infection. Clin Infect Dis 2005;40:1855-7. [Crossref] [PubMed]
- Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677-86. [Crossref] [PubMed]
- Riedel S, Melendez JH, An AT, et al. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am J Clin Pathol 2011;135:182-9. [Crossref] [PubMed]
- Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a Marker of Etiology in Adults Hospitalized With Community-Acquired Pneumonia. Clin Infect Dis 2017;65:183-90. [Crossref] [PubMed]
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018;18:95-107. [Crossref] [PubMed]
- Vincent JL, Quintairos E. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 2016;20:257. [Crossref] [PubMed]
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. [Crossref] [PubMed]
- Liu D, Xie L, Zhao H, et al. Prognostic value of mid-regional pro-adrenomedullin (MR-proADM) in patients with community-acquired pneumonia: a systematic review and meta-analysis. BMC Infect Dis 2016;16:232. [Crossref] [PubMed]
- Vigué B, Leblanc PE, Moati F, et al. Mid-regional pro-adrenomedullin (MR-proADM), a marker of positive fluid balance in critically ill patients: results of the ENVOL study. Crit Care 2016;20:363. [Crossref] [PubMed]
- Pereira JM, Azevedo A, Basílio C, et al. Mid-regional proadrenomedullin: An early marker of response in critically ill patients with severe community-acquired pneumonia? Rev Port Pneumol 2006;2016:308-14. [PubMed]
- Ferrer M, Travierso C, Cilloniz C, et al. Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS One 2018;13. [Crossref] [PubMed]
- Liapikou A, Ferrer M, Polverino E, et al. Severe community-acquired pneumonia: validation of the Infectious Diseases Society of America/American Thoracic Society guidelines to predict an intensive care unit admission. Clin Infect Dis 2009;48:377-85. [Crossref] [PubMed]
- Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996;275:134-41. [Crossref] [PubMed]
- Phua J, See KC, Chan YH, et al. Validation and clinical implications of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Thorax 2009;64:598-603. [Crossref] [PubMed]
- Chalmers JD, Taylor JK, Mandal P, et al. Validation of the Infectious Diseases Society of America/American Thoratic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis 2011;53:503-11. [Crossref] [PubMed]
- Ewig S, Woodhead M, Torres A. Towards a sensible comprehension of severe community-acquired pneumonia. Intensive Care Med 2011;37:214-23. [Crossref] [PubMed]
- Salih W, Schembri S, Chalmers JD. Simplification of the IDSA/ATS criteria for severe CAP using meta-analysis and observational data. Eur Respir J 2014;43:842-51. [Crossref] [PubMed]
- Restrepo MI, Mortensen EM, Rello J, et al. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest 2010;137:552-7. [Crossref] [PubMed]
- Renaud B, Santin A, Coma E, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med 2009;37:2867-74. [Crossref] [PubMed]
- Phua J, Ngerng WJ, Lim TK. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J 2010;36:826-33. [Crossref] [PubMed]
- ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- PRISM Investigators, Rowan KM, Angus DC, et al. Early, Goal-Directed Therapy for Septic Shock - A Patient-Level Meta-Analysis. N Engl J Med 2017;376:2223-34. [Crossref] [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [Crossref] [PubMed]
- ARISE Investigators. ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Torres A, Lee N, Cilloniz C, et al. Laboratory diagnosis of pneumonia in the molecular age. Eur Respir J 2016;48:1764-78. [Crossref] [PubMed]
- Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014;42:1749-55. [Crossref] [PubMed]
- Lee JS, Giesler DL, Gellad WF, et al. Antibiotic Therapy for Adults Hospitalized With Community-Acquired Pneumonia: A Systematic Review. JAMA 2016;315:593-602. [Crossref] [PubMed]
- Torres A, Blasi F, Peetermans WE, et al. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis 2014;33:1065-79. [Crossref] [PubMed]
- Menéndez R, Torres A, Reyes S, et al. Initial management of pneumonia and sepsis: factors associated with improved outcome. Eur Respir J 2012;39:156-62. [Crossref] [PubMed]
- Amaro R, Sellarés J, Polverino E, et al. Antibiotic therapy prior to hospital admission is associated with reduced septic shock and need for mechanical ventilation in patients with community-acquired pneumonia. J Infect 2017;74:442-9. [Crossref] [PubMed]
- Mortensen EM, Halm EA, Pugh MJ, et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA 2014;311:2199-208. [Crossref] [PubMed]
- Restrepo MI, Mortensen EM, Waterer GW, et al. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J 2009;33:153-9. [Crossref] [PubMed]
- Asadi L, Sligl WI, Eurich DT, et al. Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis 2012;55:371-80. [Crossref] [PubMed]
- Martin-Loeches I, Lisboa T, Rodriguez A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med 2010;36:612-20. [Crossref] [PubMed]
- Stern A, Skalsky K, Avni T, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev 2017;12. [PubMed]
- Welte T, Dellinger RP, Ebelt H, et al. Concept for a study design in patients with severe community-acquired pneumonia: A randomised controlled trial with a novel IGM-enriched immunoglobulin preparation - The CIGMA study. Respir Med 2015;109:758-67. [Crossref] [PubMed]
Cite this article as: Ceccato A, Torres A. Sepsis and community-acquired pneumonia. Ann Res Hosp 2018;2:7.