
Rhythmicity as an important regulatory factor in complex biological systems: introduction to chronopharmacology
Introduction
The most famous quote of the ancient Greek philosopher Heraclitus (approximately 535–475 BC) is “everything flows, nothing ever stays the same”, which describes the foundation of his philosophical approach of cosmology and life (1). According to the philosopher, all aspects of the world and universe, including life, are subject to continuous, (in many cases cyclic) changes, as a consequence of the balance attempted to be established between opposing forces; another Heraclitus quote “you can never enter twice into the same river” highlights in a very illustrative manner this concept. On the contrary, our scientific perception on living systems is being dominated by the work of the French physiologist Bernard in 1865, who claimed that the stability of the internal environment, the “milieu intérieur”, is the condition for the free and independent life (2), and 60 years later by Cannon (who introduced the term homeostasis to describe the state of steady internal conditions maintained by living things), who highlighted the importance of stability for life preservation (3).
Nowadays, advancements in research techniques, methodologies and our deeper understanding of critical concepts of biology enable us to realize that complex living systems are most likely governed by a combination of both points of view. While living systems possess ideal functional states and have developed biological mechanisms to support and preserve these states, and to some extent counteract opposing conditions, at the same time, many of these biological mechanisms are characterised by a continuous, usually cyclical, mobility. This creates a temporal pattern (or rhythm), being repeated on either a daily basis (circadian rhythm), multiple times within each day (ultradian rhythms) or having a period longer than 24 hours (infradian rhythms).
Recent evidence highlights the importance of the different aspects of bio-rhythmicity for homeostasis and creates the need for a closer investigation on how the synchronicity between the rhythms of interacting biological processes reassures homeostasis or acts as a substrate for the development of disease or even defines the efficacy of pharmacological interventions in the context of pathology. In this perspective, I will try to highlight a few crucial concepts on the significance, and the clinical implications, of bio-rhythmicity.
Understanding the concept of bio-rhythmicity
The concept of bio-rhythmicity (i.e., the periodic oscillation of biomolecular processes like for instance gene expression or biochemical reactions or cell proliferation or secretion of hormones etc.) has been identified many decades ago and has been considered as an inherent phenomenon of biological systems, which relates to their ability to adapt to the unstable external environment (4). From an empirical point of view, this notion should be (at least partly) true; the environmental conditions change periodically (like the circadian alternation between the presence and absence of sunlight or the infradian changes in mean temperature or the ultradian activation of the stress system in response to threatening cues), and biological systems should be capable to retain or re-establish their homeostasis despite that.
From a logical point of view, though, another important reason also exists; every biological system needs to manage its energy balance the most efficient way possible, which means (among others) the proper distribution of resources (and the mobilization of corresponding bodily states) to support functions most appropriate for a given time. In many occasions these functions are environment-dependent, but in others they depend on individual preferences or the smooth succession between different internal states. Some examples include food intake, sexual behaviour, goal-oriented decision making, but also the mobilization of the processes involved in digestion (which should follow food intake) or long-term adaptation (following a stress response).
Thus, a complex biological system needs to integrate external cues, internal states and individual behaviors into a functional framework, which ensures efficiency (i.e., contextual quick and successful responses) and maximizes the chances for survival. Apparently, this functional framework seems to involve the registration of certain biological processes with certain time periods, and this registration can be differentially sensitive to temporal modifications.
Neurohormonal networks orchestrate bio-rhythmicity: the master pacemaker and the example of the hypothalamic-pituitary-adrenal (HPA) axis
Bio-rhythmicity in the mammalian body seems to involve pacemakers in multiple tissues (for instance the brain, heart, liver, muscles, hormone-secretory organs etc.), which are coordinated by a master pacemaker, located in the central nervous system (CNS). This master pacemaker is traditionally considered to be the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, which uses the light-dependent input of retina as its driving cue to adjust its rhythmic electrical activity and modulate gene expression, protein modifications and secretion in the brain and other target, peripheral tissues (5). A very well described example of such a network between the SCN and other tissues is the HPA axis controlling the secretion of a vital class of hormones, the glucocorticoids [corticosteroids (CORTs)].
CORTs are characterized by a fascinating, complex bio-rhythm, which has a circadian as well as an underlying ultradian component, created by the interplay between central and peripheral pacemakers. The circadian component of CORT rhythmicity consists of a huge peak of the hormonal levels occurring 2–3 hours prior the start of the active phase of each day (for instance prior waking up from the night sleep in human or the morning sleep in rodents), and the subsequent gradual fall until the circadian cycle resets. For this diurnal variation SCN differentially reduces its inhibitory input to the neighbouring paraventricular nucleus and median eminence, which in turn differentially intensify the secretion of corticotropin-releasing hormone (CRH) to the hypophyseal portal circulation, which in turn differentially upregulates corticotrophin [adrenocorticotropic hormone (ACTH)] secretion by the anterior pituitary, which finally travels via the systemic circulation to adrenal glands and differentially stimulates CORT biosynthesis and immediate release.
Underlying CORT’s circadian rhythm is another, more dynamic one, characterized by frequent hormonal oscillations, occurring multiple times daily. This is the ultradian rhythm of the hormone, conserved among mammalian species, mainly controlled by the ACTH positive feedforward—CORT negative feedback interactions with built-in delays between the anterior pituitary and adrenal cortex. Thus, a triangular system of in-between interactions forms the core part of a network, (influenced by multiple other, secondary, peripheral and central stimuli) that closely monitors the temporal pattern by which CORT is released on a daily basis (6).
Aside the HPA, other fundamental neurohormonal networks, characterized by equally complex bio-rhythmicity, also exist; the hypothalamic-pituitary-thyroid axis, regulating the secretion of thyroid hormones (implicated in energy balance regulation), the hypothalamic-pituitary-gonadal axis, regulating the secretion of sex hormones (implicated in development, cell proliferation, regulation of various anabolic biochemical reactions, brain physiology including sexual behaviour, and physiology of reproduction), other hypothalamic-pituitary interactions modulating the secretion of growth hormone (implicated in bone and soft tissue development and physiology), vasopressin (regulating the balance of fluids), prolactin or oxytocin, and hypothalamic-pineal gland interactions modulating the secretion of melatonin (which regulates the sleep-awaking cycles and also exhibits cytoprotective properties) (7).
Multiple peripheral biological clocks exist: examples from the metabolism and the immune system
Aside the classical neurohormonal networks, mentioned before, accumulating evidence now suggest that other peripheral tissues contain biological pacemaker mechanisms, sources of bio-rhythmicity. Two examples include the hepatic metabolism and the respiratory immune system.
Very recently, Zhu et al. (8) utilized an eigenvalue/pencil approach, previously developed for spectrum analysis in the digital signal processing field, to identify oscillations in gene expression of over 18,000 genes from a high-resolution hepatic gene expression microarray (temporal resolution 1 h, total period covered 48 h) of mice kept under constant darkness. This approach unraveled hepatic gene expression oscillations characterised by ultradian rhythms (with a pulse frequency of 12, 8, 6 or 4 h) some of which, like CORTs, had a superimposed circadian one. By focusing on the genes exhibiting a 12 h cycle (i.e., circatidal rhythm), the researchers were able to demonstrate that this bio-rhythmicity is created by a cell-autonomous clock, independent from the circadian one. Most of the genes involved, express proteins localized in both, the endoplasmic reticulum (ER) and mitochondria, participating in cellular metabolism and metabolic stress confrontation. The data support the notion for a strong relationship between the mammalian 12 h clock system and the interplay between the ER and mitochondria.
On another note, Gibbs et al. (9) showed that a local pulmonary clock regulates dynamic changes in response to in vivo bacterial challenge, and that a neutrophil chemokine (CXCL5) is clock-controlled, mediating a diurnal variation of the magnitude of lung inflammation. Moreover, the study revealed the presence of a local circadian circuit regulating bronchiolar glucocorticoid receptor (GR) function on CXCL5 gene expression by imposing time-of-day-varying epigenomic changes affecting GR’s recruitment on the process. The authors suggest that, under physiological conditions, CXCL5 is regulated by the interplay between the local circadian bronchiolar clock and the adrenal-originating repressive CORT signals, resulting in clock-regulated responses to endotoxin and bacterial infection. Loss of that interplay (by a dysregulation of either the local or the systemic pacemakers due to external stimulation, like smoking, disease or therapeutic manipulations) could affect the efficacy of the innate inflammatory responses.
Bio-rhythmicity and brain function
The concept of bio-rhythmicity has been long appreciated in the context of brain function. Large-scale neural networks create a resultant electrical activity, which oscillates periodically, and is related to the various fundamental cognitive processes mediated by CNS. For instance, electroencephalographic (EEG) recordings have determined the presence of fronto-central frequencies over 22.5 Hz and parietal alpha activity which are strongly modulated by a 3–4 h ultradian periodicity and have been associated with arousal and the circadian sleep/ awake cycle (10). Changes in the oscillatory characteristics of these networks could result to neuropsychiatric symptomatology (11) and disease. A prominent example involves the interplay between cortical, striatal and extra-striatal neurons of the basal ganglia, like the subthalamic nucleus (STN), which control habitual-dependent motion. The core part of this network involves a triangular system in which cortical neurons directly innervate striatum and STN, while striatal neurons also (indirectly) communicate with STN. Under physiological conditions, cortical oscillatory stimulation of striatum is suppressed by endo-striatal inhibitory processing, leading to a silent striatal-STN communication. By contrast, STN neurons respond to cortical rhythmicity with their own rhythmical excitation. Dopaminergic neurodegeneration downregulates the endo-striatal suppression of the oscillatory cortical input (un-silencing STN-striatal interplay) and increases the oscillatory coupling between STN and cortical neurons, potentially impairing motor processing (12).
Brain function is not only modulated by the homeostasis between inter-neuronal dynamics, but also by the homeostasis in the rhythmicity of non-neural signals. Over the past 2–3 decades, for instance, we have progressively gathered multiple sources of evidence, derived by multimodal in silico, in vitro, in vivo, preclinical and clinical, interventional and observational approaches, that not only CORTs crucially affect brain function, but their endogenous, naturally complex rhythm constitutes a neurobiological signal per se. The brain is exposed to both aspects of the complex CORT rhythm, and brain cells have developed molecular mechanisms to respond to both, the circadian and the ultradian cues related to CORT rhythmicity. The latter constitutes, therefore, a complex chronobiological signal able to concurrently regulate circadian processes, like the daily homeostasis of energy balance in brain cells, neural survivability, and multi-neuronal network dynamics, affecting mood, sleep behaviour and alertness, and ultradian processes, like episodes of neurotransmission controlling synaptic and dendritic plasticity, affecting memory, emotional processing or the readiness for initiating a stress response (13).
Bio-rhythmicity, stress adaptation, ageing and disease
Nowadays we know that transient changes in bio-rhythmicity facilitate acutely the successful mobilization of the stress system, and the long-term neurobehavioural adaptations following the stressful insult. We have also established that long lasting changes to various forms of bio-rhythmicity are associated to the process of ageing, like the phase advance and reduced amplitudes of the diurnal variation of temperature, melatonin and CORTs (14). Moreover, we also reasonably suspect that persistent deviations from the physiological rhythmicity of various biological phenomena, or the desynchronization between interacting bio-rhythms, could result to disease.
For instance, the ultradian rhythm of CORTs (which constitute key hormones for the coordination of the stress system responses) ensures that the mammalian body will be capable of expressing effective stress responses (if required) throughout the day; without the pulsatile component in CORTs’ rhythmicity, these responses could be even stronger during the period surrounding the circadian peak, but their effectiveness would drop dramatically for a very long-time coinciding with the circadian trough of the hormone. Moreover, a shift in the CORT rhythm from a rapid oscillation, under basal conditions, to a pulse of higher amplitude and longer duration, under stressful conditions, would upregulate the activation of (and consequently the cell signaling events induced by) certain variants of CORT-sensitive receptors in the brain, namely membranous mineralocorticoid receptors and GRs, which support cellular functions related to the proper initial brain response to stress and to the long-term cognitive modifications required for an effective confrontation of similar insults in the future (15).
On another note, bio-rhythmicity is reciprocally affected by pathology. Aside the example of Parkinson’s disease, which is characterised by the dysrhythmicity between the nodes of the triangular neural system controlling habitual-like motion, mentioned before (12), other examples also exist. Disrupted CORT rhythmicity is present in a great number of neuropsychiatric diseases; Alzheimer’s disease, depression, traumatic brain injury, stroke, fibromyalgia and chronic fatigue syndrome, chronic insomnia and post-traumatic stress disorder (15). Tumor cells and tissues either exhibit or impose systematically various deviations from physiological circadian rhythms; diminished amplitude, phase shifts, period changes, and erratic peaks and troughs have been reported in various components of the endocrine, metabolic, immune systems, as well as the sleep-awake cycles. In turn, such a circadian dysrhythmicity might facilitate tumor growth by changing the metabolic, endocrine and immunological environment in which tumor cells reside (16). Chronic circadian desynchronization, like that observed after chronic reversal of the external light-dark cycle, decreases the survival of animals with cardiomyopathic heart disease (17). And the list of examples can go on.
Epilogue: therapeutic implications of bio-rhythmicity
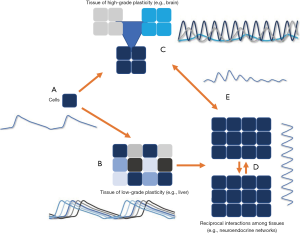
Summarizing, what we’ve established so far is the fact that bio-rhythmicity is an inherent phenomenon of complex living systems, created by the interplay among different infra- and supracellular mechanisms (Figure 1) and influenced by internal or external cues. The mammalian body possesses a master pacemaker, the SCN located in the anterior hypothalamus of the CNS, while the rest of the brain, as well as many peripheral organs also have local clock mechanisms. The master pacemaker interacts with many of these local clocks in the context of various biological processes, giving them a resultant form of bio-rhythmicity. The latter regulates important aspects of cognitive, metabolic, immunological homeostasis, and the body’s effective responsivity to stressful stimuli and noxious insults. Moreover, sustained dysregulation of bio-rhythmicity is associated with the process of ageing and disease. The concept of bio-rhythmicity gave birth to the field of chronobiology, which progressively gains increasing scientific attention. The same should happen with the field of chronopharmacology (18).
According to the classical pharmacological views, the presence in high abundance of a biologically relevant stimulus (being for instance a natural hormone or an environmental factor, or even an artificially synthesized chemical compound) casts significant and detectable changes upon a biological system, when comparing it with the state of the stimulus’ absence. Nevertheless, the states of high abundance and total absence are extreme conditions; as I’ve supported so far, biological systems are used to function in a grey zone, where the various stimuli dynamically alternate between becoming relatively scarce and relatively abundant and have developed molecular mechanisms to respond appropriately. Moreover, multiple stimuli affect every biological system at any given timepoint, and many of their effects share common downstream pathways, having (either parametric or even non-parametric) additive or nullifying effects. In other words, it not only matters if a stimulus is present or absent, but (equally important) when is it present and when absent.
In this context, the frequency and duration by which a stimulus is in position to impose a certain effect on a biological system (let’s call this feature “effective presence”) is of paramount importance. For instance, if a stimulus increases the frequency of its effective presence in a given biological system, this could potentially change its effects on this system by either (I) acting upon a cellular/molecular substrate which didn’t have the time to reset from that stimulus’ prior effects (or other stimuli’s effects with common downstream pathways) or (II) increasing the probability of acting concurrently with other stimuli on common downstream pathways. Moreover, if a stimulus increases the duration of its effective presence in a given biological system, this could again potentially change its effects on this system by either (I) maximizing its cellular/ molecular substrate’s response, or (II) causing adaptive down-regulation of the substrate’s response, or (III) recruiting additional mechanisms, or again (IV) increasing the probability of acting concurrently with other stimuli on common downstream pathways.
Examples on the importance of chronopharmacological parameters for drugs’ efficacy already exist: we’ve earlier mentioned that the respiratory system applies a circadian variation on the recruitment of GR to regulate inflammatory responses. Therefore, GR-acting anti-inflammatory agents (like dexamethasone) are not equally effective throughout the day. Experimental data also support that the drug-induced toxicity of anticancer agents, like cyclophosphamide, show circadian variation depending on the functional status of the CLOCK/BMAL1, a heterodimer DNA binding protein involved in the transcription of several genes implicated in the circadian clock mechanisms in mammals (19). Similarly, time-controlled interventions might improve the therapeutic outcome in hypertension and congestive heart disease (20). Furthermore, it has been established that insulin sensitivity in patients with non-insulin dependent diabetes exhibits a diurnal variation, decreasing during the night and increasing during the day (21). Finally, recent evidence demonstrates that changes in the temporal mode of CORT replacement therapy differentially impacts sleep quality, working memory performance and the connectivity between corticolimbic regions modulating mood and emotional processing (22). Future studies should systematically address all these emerging issues towards improving the efficacy of various established therapeutic agents and open new pathways for novel translational research. Moreover, the wide spectrum of the clinical implications (Table 1) of bio-rhythmicity should be systematically studied.

Full table
Acknowledgements
The author would like to express his gratitude to Bodossaki Foundation (https://www.bodossaki.gr/en/) for supporting the research on modelling glucocorticoid biorhythmicity.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kirk GS. Heraclitus: the cosmic fragments. Cambridge: Cambridge University Press, 1954:381.
- Bernard MC. Leçons sur la chaleur animale, sur les effets de la chaleur et sur la fièvre. Paris: Librairie J.-B. Baillière et Fils, 1876.
- Cannon WB. The wisdom of the body (2nd ed.). Oxford: Norton & Co, 1939.
- Hobson A. Sleep and biorhythmicity. Science 1969;165:932-3. [Crossref] [PubMed]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 2003;4:649-61. [Crossref] [PubMed]
- Russell GM, Kalafatakis K, Lightman SL. The importance of biological oscillators for hypothalamic-pituitary-adrenal activity and tissue glucocorticoid response: coordinating stress and neurobehavioural adaptation. J Neuroendocrinol 2015;27:378-88. [Crossref] [PubMed]
- Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia 1993;49:654-64. [Crossref] [PubMed]
- Zhu B, Zhang Q, Pan Y, et al. A Cell-Autonomous Mammalian 12 hr Clock Coordinates Metabolic and Stress Rhythms. Cell Metab 2017;25:1305-19.e9. [Crossref] [PubMed]
- Gibbs J, Ince L, Matthews L, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 2014;20:919-26. [Crossref] [PubMed]
- Chapotot F, Jouny C, Muzet A, et al. High frequency waking EEG: reflection of a slow ultradian rhythm in daytime arousal. Neuroreport 2000;11:2223-7. [Crossref] [PubMed]
- Winkelmann A, Maggio N, Eller J, et al. Changes in neural network homeostasis trigger neuropsychiatric symptoms. J Clin Invest 2014;124:696-711. [Crossref] [PubMed]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 2007;30:357-64. [Crossref] [PubMed]
- Kalafatakis K, Russell GM, Lightman SL. Does circadian and ultradian glucocorticoid exposure affect the brain? Eur J Endocrinol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci 2012;13:325-35. [Crossref] [PubMed]
- Kalafatakis K, Russell GM, Zarros A, et al. Temporal control of glucocorticoid neurodynamics and its relevance for brain homeostasis, neuropathology and glucocorticoid-based therapeutics. Neurosci Biobehav Rev 2016;61:12-25. [Crossref] [PubMed]
- Fu L, Pelicano H, Liu J, et al. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002;111:41-50. [Crossref] [PubMed]
- Penev PD, Kolker DE, Zee PC, et al. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol 1998;275:H2334-7. [PubMed]
- Dallmann R, Brown SA, Gachon F. Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol 2014;54:339-61. [Crossref] [PubMed]
- Gorbacheva VY, Kondratov RV, Zhang R, et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A 2005;102:3407-12. [Crossref] [PubMed]
- Lemmer B. The importance of circadian rhythms on drug response in hypertension and coronary heart disease--from mice and man. Pharmacol Ther 2006;111:629-51. [Crossref] [PubMed]
- Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996;45:1044-50. [Crossref] [PubMed]
- Kalafatakis K, Russell GM, Harmer CJ, et al. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc Natl Acad Sci U S A 2018;115:E4091-100. [Crossref] [PubMed]
Cite this article as: Kalafatakis K. Rhythmicity as an important regulatory factor in complex biological systems: introduction to chronopharmacology. Ann Res Hosp 2018;2:14.


