
Association of periodontal disease indices with risk of gastric adenocarcinoma
Introduction
Gastric cancer (GC) consists the fourth most common cancer worldwide by incidence, however, its rates have substantially decreased over the past century due to changes in environmental risk factors (1). Epidemiological observations suggest that H. pylori infection, heavy smoking, alcohol consumption, dietary habits and genetic factors play important roles in GC pathogenesis (2,3). Periodontal disease (PD), gingivitis and periodontitis affect many people in Greece (4), although recent studies have not been carried out.
A recent epidemiological research has linked periodontal pathogens to several systemic diseases, including cardiovascular disease, diabetes mellitus, respiratory disease, and systemic infections, associations possibly mediated through markers of systemic infection and inflammation (5). It has also been suggested a possible association between PD and cancer risk in different organs, most notably in the oral cavity, upper gastrointestinal system, lung, and pancreas (6-13). Poor oral health or hygiene, as indicators of PD, are possible risk factors for cancer, and significant associations have been recorded in different organs (6,7,12,14) and stomach, overall and by its anatomic subtypes that is cardia and non-cardia (9,15). Prospective epidemiological studies have recorded such associations between tooth loss, as another PD indicator, and the risk of gastric non-cardia adenocarcinoma (9,15-17). It has been hypothesized that PD increases the risk of gastric non-cardia adenocarcinoma through alterations in the oral bacterial flora and subsequent chronic systemic inflammation (9,15). However, to our knowledge no previous studies have objectively evaluated the role of PD in GC or precancerous lesions.
The aim of the current case-control study was to investigate the possible association between PD parameters and GC in a Greek adult population.
Methods
Study sample
The study sample consisted of 724 individuals (426 males and 298 females). Cases and controls, were selected from a private dental and two private medical practices, were filled in a health questionnaire and were undergone an oral clinical examination.
Patient selection criteria
GC patients (cases) and controls were recruited from the same population. They should have a mean of 20 natural teeth, since large numbers of missing teeth could lead to over- or under-estimate the PD variables and the possible associations that were under consideration.
Clinical criteria of established periodontitis (18), which referred to at least 2 teeth with clinical attachment loss (CAL) ≥6 mm and more than one site with probing pocket depth (PPD) ≥5 mm were used for selection of participants.
The participants should not have been received any periodontal treatment, conservative or surgical during the previous six months or prescription of anti-inflammatory or systemic antibiotics or other systemic drugs the previous six weeks (19).
In order to avoid as much as possible potential confounding influences on the study parameters, participants with acute gastric infections, cardiovascular diseases, diabetes mellitus, rheumatoid arthritis, immuno-suppressed patients because of haematological malignancy or recent transplantation and those who received treatment for the mentioned diseases, liver cirrhosis and concurrent medication with general glucocorticoids were excluded from the study. They also excluded patients with advanced GC under medical treatment, patients with gastric metastases of a primary focus at a different location, patients diagnosed with cardia-adenocarcinoma or other focuses in the region of head-neck-thorax-carcinogenesis field theory (20), and patients with prior gastric surgery, current use of anticoagulants, with active gastrointestinal bleeding and having had or suspected to have oesophageal varices. The mentioned conditions were determined because of potential effects on the oral and periodontal tissues. Hospital patients were not included or patients with several location of cancer in which smoking are considered as a proven risk factor such as larynx cancer, nasopharyngeal cancer, etc.
The variable ‘genetic predisposition’ was defined according to the relative risk of GC in cases in which was found to be elevated for all first-degree relatives, i.e., siblings and parents of the participants of the study (21). Previous H. pylori infection was detected by examination of the participants’ medical files. For this purpose a rapid gastric urease test kit (Lencomm Trade International®, Poland) was used in order to an individual to be considered positive for H pylori.
The patients’ group was consisted of individuals in which the diagnosis of non-cardia adenocarcinoma was set initially by a histological examination during the endoscopic procedure and they had been given instructions regarding their oral hygiene the after diagnosis and before the application of any treatment method, such as surgery, radiotherapy or chemotherapy.
Controls group selection was carried out by the friendly and collegial environment of cases group in an effort to control potential confounders. Both groups, cases and controls, were matched referring to age, gender and smoking status (current/previous smokers and never smokers). Based on the observation that epidemiological studies have identified age, smoking history and gender as the main established risk factors for periodontitis as covariates (5), patients with GC and controls were matched for these criteria. For each GC patient, two controls with the same age (±3 years) and the same socio-economic status was selected. For ten of the 248 GC patients, it was not possible to find controls who matched the selected criteria.
The research was performed between January 2015 and March 2016.
Oral clinical examination
The participants, cases and controls were undergone an oral clinical examination by a well-trained and calibrated dentist at the mentioned private practices. The clinical parameters that assessed were the following periodontal indices: gingival index (GI), PPD, CAL and bleeding on probing (BOP) were measured by a William’s 12 PCP probe (PCP 10-SE, Hu-Friedy Mfg. Co. Inc., Chicago, IL, USA) at six sites (facial, lingual, disto-facial, mesio-facial, disto-lingual and mesio-lingual) for each tooth, except for the 3rd molars and the remaining roots. Gingivitis severity was classified as follows: score 0—normal situation of gingival tissue/mild inflammation, which corresponds to Löe and Silness (22) classification as score 0 and 1; score 1—moderate/severe inflammatory reaction, which corresponds to Löe and Silness (22) classification as score 2 and 3. The presence of PPD was classified as follows (23): score 0—moderate periodontal pockets, 4–6.0 mm; score 1—advanced periodontal pockets, >6.0 mm. CAL severity classified as follows (24): score 0—mild, 1–2.0 mm of attachment loss; score 1—moderate/severe, ≥3.0 mm of attachment loss. The estimation for PPD and CAL measurements concerned the immediate full millimetre. The presence/absence of BOP was classified as follows: score 0—absence of BOP; score 1—presence of BOP and recorded as positive if it was occurred within 15 seconds of probing.
Questionnaire
Cases and controls were completed a self-administered questionnaire that included variables such as age, gender, smoking status (active, former/no-smokers), socio-economic and educational level and data regarding the medical history of them with reference to medication, several chronic systemic disorders and their dental follow-up frequency.
A randomly chosen sample of 145 (20%) individuals was re-examined clinically by the same dentist after 3 weeks in order to estimate the intra-examiner variance. After consideration of the code numbers of the double examined individuals no differences were recorded between the 1st and the 2nd clinical assessment (Cohen’s Kappa =0.97) whereas for the mentioned time period no oral hygiene instructions were given to the participants.
Ethical consideration
In Greece only experimental studies must be reviewed and approved by authorized committees (Greek Dental Associations, Ministry of Health, etc.). The current case-control study was a retrospective non-experimental one. Individuals who agreed to participate in the present study signed an informed consent form.
Statistical analysis
The worst values of GI, PPD and CAL at the six sites per tooth and the presence/absence of BOP were recorded and classified as dichotomous variables for each case and control. Males participants were coded as 1, current and former smokers were coded as 1, individuals with a high socio-economic (income/monthly equivalent to or above €1,000) and educational level (graduated from University/College) were coded as 0, individuals that reported a genetic predisposition for GC, history of previous histological diagnosis of H. pylori infection and a regular dental follow-up were coded as 1. Univariate analysis was performed to assess the association between the independent variables examined and the GC risk, separately, by using the chi-square test model. Multivariate regression analysis model was carried out to assess the associations between the dependent variable, GC, and independent ones that were determined by the enter method. Adjusted (odds ratios) OR’s and 95% CI were calculated as well. According to the last step of the model, the independent variables were included to stepwise method in order to assess gradually the variables that showed significant associations with the dependent one. For controlling the influence of possible con-founders, the statistical method Cohran’s and Mantel-Haenszel’s was carried out, in order to avoid possible biased secondary associations. Statistical analysis was performed using the statistical package of SPSS ver.17.0. A P value less than 5% (P<0.05) was considered to be statistically significant.
Results
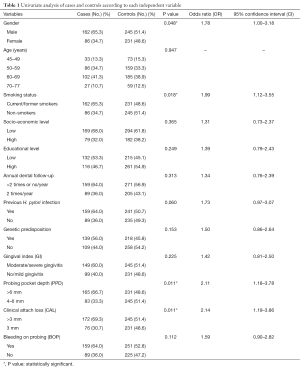
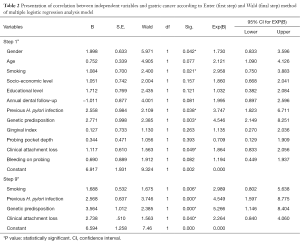
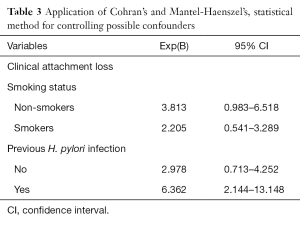
The mean age of the cases and controls was 58.4±3.7 years. Table 1 presents univariate analysis of cases and controls regarding the examined variables. PPD, CAL, BOP, male gender and smoking were statistically significantly associated with GC risk. Table 1 also presents unadjusted OR’s and 95% CI. After performance of the first method (step 1a) of the regression model it was found that male gender, smoking, previous H. pylori infection, genetic predisposition and CAL were significantly associated with GC risk (Table 2). Table 2 also presents adjusted OR’s with 95% CI. The final method (step 9a) showed that smoking, previous H. pylori infection, genetic predisposition and CAL were significantly associated with GC risk. CAL was also significantly associated with CG risk after adjusting for confounders, such as smoking and previous H. pylori infection (Table 3).
Full table
Full table
Full table
Discussion
GC is the fourth most common cancer and second highest in mortality worldwide (1,25). Despite the fact that environmental and lifestyle factors are considered as risk factors of GC (26) they could only explain less than 60% of GC incidence (27).
The possible influence of male gender as a cancer risk factor is known (28-30), however it is considered as a confounder during statistical analysis. No association was recorded between gender and GC risk, finding that was in accordance with those from previous reports (31,32). Similarly, age is considered as a confounder, although older individuals are in a higher risk for total cancer, GC (33), initiation and progression of PD (34). No association was found in the present study between age and GC risk, finding that was in agreement with those from previous investigations (31,32). Socioeconomic level is also a crucial confounder, however it has been proven its possible role as a GC risk factor (35,36) finding that was not confirmed by the current study.
It has been suggested that bacterial infections are linked with GC through the induction of gastric chronic inflammation (37). In the classic example H. pylori infection triggers a chronic inflammation and leads to gastric non-cardia adenocarcinoma (38). Epidemiological reports (39,40) have shown that dental plaque and saliva are reservoirs for H. pylori, whereas they also have found that deep periodontal pockets were positive for H. pylori (41) and that H. pylori was detected in more than 50% of patients with periodontitis who harboured H. pylori in their stomachs (42,43). On the other hand, conflicting reports exist in the literature regarding the presence of H. pylori in the oral cavity and its relation to its presence in the stomach (44,45). The present study recorded a significant association between previous H. pylori infection and CG risk.
Previous reports have not investigated the possible association between the role of educational level and GC risk, however it is supposed that high-educated individuals take care of their own oral hygiene more than low-educated ones (46,47). No association was recorded between educational level and GC risk in the current study.
Smoking is considered as a causal risk factor of total cancer, including GC (26) and is associated with adenocarcinoma, whereas it is still remaining unknown the reasons why only a small amount of smokers develop GC (27). The current report confirmed its role as a causal risk factor. On the other hand, smoking is considered as a risk factor for PD development and progression (48,49) and a proven confounder as well.
The nature of the genetic factors has not been well-studied and, outside of a few rare cancer syndromes, the genes involved have not been identified. Having a first-degree relative with GC is a consistent risk factor for GC, although the magnitude of the OR associated with a positive family history varies with the ethnic group and with the geographic region (2). The results of the current study confirmed such an association.
Epidemiological studies with various samples have investigated the possible associations of self-reported tooth loss or poor oral health/hygiene with the risk of GC and conflicting results were recorded (8,14-17,32,50-52). Those conflicting findings could be attributed to the methodological differences in the criteria used to define PD and oral health, differences in study sample populations, difficulties in distinguishing gastric cardia and non-cardia cancers, differences in risk factors in high and low incidence areas, or chance findings (type I statistical bias).
Several mechanisms have been suggested to explain the possible association between oral health and upper gastrointestinal tract cancers which may be related to the local activation of carcinogens in tobacco, alcohol, or the diet, such as acetaldehyde (53) or nitrosamines (9), or based on carcinogenesis field theory (20). Poor oral health can also increase the production of the mentioned products (54). The use of oral antiseptics (e.g., chlorhexidine) decreases salivary nitrosation (55) and may also reduce the production of other metabolites. Alterations of dietary pattern, such as decreased fruits and vegetable intake secondary to tooth loss (56) may also predispose to oral and upper gastrointestinal cancers.
BOP was not significantly associated with GC risk, although in a similar case-control study (32) a positive association was observed between BOP and gastric precancerous lesions, independent of other risk and confounding factors. BOP is considered as an objective indicator for detecting gingival inflammation (57) and is associated with active gingivitis and periodontitis.
A growing body of epidemiological and laboratory evidence has emerged showing that longstanding inflammation promotes tumour development, growth and progression. This finding suggests that chronic inflammation is a risk or prerequisite factor for the development of a number of human malignancies, including liver, colon, stomach, lung carcinomas, etc. (58).
Several oral pathogens have been related to chronic systemic inflammation (59), which has been associated with increased risk of GC due to increased secretion of interleukins 1a and 1b (Il-1a, Il-1b), tumour necrosis factor-alpha (TNF-a) and C-reactive protein (CRP) levels (60-64).
No association was found between PPD and GC risk. Similar reports have not been carried out, whereas the majority of the available studies were prospective and based on questionnaires and self-reported data.
CAL, which estimates the severity of PD (24) and higher CAL with time is the best single indication that periodontitis has probably recurred (65), was found to be significantly associated with GC risk. This observation suggests that chronic inflammation, as PD, could be a risk or a prerequisite factor for the development of a number of human malignancies, as already has mentioned.
Several potential limitations should also be noted. First, the time sequence of oral health conditions, behaviours and GC lesions cannot be directly addressed in the current study.
However, it is not likely that subjects reported their oral health behaviours differently according to their disease status, as the disease status was determined based on biopsy findings previously to the interview. Second, although we cannot exclude the possibility of potential selection bias, it is not likely that cases with severe PD preferentially volunteered to be included in the study, since the issues examined were not known by the participants and participants were unaware of their PD status at the time of recruitment and oral health examination. Another practical problem is the accuracy definition of PD which is essential to establish on reliable and reproductive indices (66).
It is important to highlight that the decision on including older individuals who have at least 20 remaining natural teeth, may lead to an under-estimation of older individuals with previous PD and who may have had teeth extracted for periodontal reasons. In addition, it is essential to be noticed that there was not any chance of benchmarking between the findings of the current study with those of similar previous studies, whereas on the other hand the present study was a first attempt to approach that possible correlation in Greece.
Conclusions
In conclusion, CAL as an index for PD severity and the known risk factors such as smoking, previous H. pylori infection, and genetic predisposition were statistically significantly associated with the risk of GC development.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study needs no approval of the Institutional Review Board (IRB) as it was not an experimental one and based on questionnaires and simple dental examinations. All enrolled patients had not been examined by histological examination as the diagnosis of adenocarcinoma had been set before the study design.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003;56:1-9. [Crossref] [PubMed]
- Barr H. Gastric tumours. Medicine 2007;35:216-9. [Crossref]
- Mamai-Homata E, Polychronopoulou A, Topitsoglou V, et al. Periodontal diseases in Greek adults between 1985 and 2005-Risk indicators. Int Dent J 2010;60:293-9. [PubMed]
- Teng YT, Taylor GW, Scannapieco F, et al. Periodontal health and systemic disorders. J Can Dent Assoc 2002;68:188-92. [PubMed]
- Michaud DS, Joshipura K, Giovannucci E, et al. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst 2007;99:171-5. [Crossref] [PubMed]
- Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, et al. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr 2003;78:176-81. [PubMed]
- Hujoel PP, Drangsholt M, Spiekerman C, et al. An exploration of the periodontitis cancer association. Ann Epidemiol 2003;13:312-6. [Crossref] [PubMed]
- Abnet CC, Qiao YL, Mark SD, et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control 2001;12:847-54. [Crossref] [PubMed]
- Abnet CC, Qiao YL, Dawsey SM, et al. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol 2005;34:467-74. [Crossref] [PubMed]
- Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol 1998;34:284-91. [Crossref] [PubMed]
- Rosenquist K, Wennerberg J, Schildt EB, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol 2005;125:1327-36. [Crossref] [PubMed]
- Chrysanthakopoulos NA. Correlation between periodontal disease indices and lung cancer in Greek adults. Exp Oncol 2016;38:49-53. [PubMed]
- Michaud DS, Liu Y, Meyer M, et al. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol 2008;9:550-8. [Crossref] [PubMed]
- Abnet CC, Kamangar F, Dawsey SM, et al. Tooth loss is associated with increased risk of gastric non-cardia adeno-carcinoma in a cohort of Finnish smokers. Scand J Gastroenterol 2005;40:681-7. [Crossref] [PubMed]
- Watabe K, Nishi M, Miyake H, et al. Lifestyle and gastric cancer: a case- control study. Oncol Rep 1998;5:1191-4. [PubMed]
- Demirer T, Icli F, Uzunalimoglu O, et al. Diet and stomach cancer incidence. A case control study in Turkey. Cancer 1990;65:2344-8. [Crossref] [PubMed]
- Machtei EE, Christersson LA, Grossi SG, et al. Clinical criteria for the defini¬tion of established periodontitis. J Periodontol 1992;63:206-14. [Crossref] [PubMed]
- Machuca G, Segura-Egea JJ, Jimenez-Beato G, et al. Clinical indicators of periodontal disease in patients with coronary heart disease: A 10 years longitudinal study. Med Oral Patol Oral Cir Bucal 2012;17:e569-74. [Crossref] [PubMed]
- Rubin H. Fields and field cancerization: the preneoplastic origins of cancer: asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked by saturation density in culture. BioEssays 2011;33:224-31. [Crossref] [PubMed]
- Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer 2010;102:237-42. [Crossref] [PubMed]
- Löe H, Silness J. Periodontal disease in pregnancy. Acta Odontol Scand 1963;21:533-51. [Crossref] [PubMed]
- Russell AL. Epidemiology of periodontal disease. Int Dent J 1967;17:282-96. [PubMed]
- Wiebe CB, Putnins EE. The periodontal disease classification system of the American Academy of Periodontology-an update. J Can Dent Assoc 2000;66:594-7. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of world-wide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62. [Crossref] [PubMed]
- Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 2003;95:1404-13. [Crossref] [PubMed]
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer 2003;97:3133-75. [Crossref] [PubMed]
- Ito LS, Inoue M, Tajima K, et al. Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol 2003;13:24-31. [Crossref] [PubMed]
- Yalcin S. Nutrition and gastric cancer in Turkey- bridge between west and east. Nut Cancer 2009;61:900-2. [Crossref]
- Shakeri R, Malekzadeh R, Etemadi A, et al. Association of Tooth Loss and Oral Hygiene with Risk of Gastric Adenocarcinoma. Cancer Prev Res (Phila) 2013;6:477-82. [Crossref] [PubMed]
- Salazar CR, Francois F, Li Y, et al. Association between oral health and gastric precancerous lesions. Carcinogenesis 2012;33:399-403. [Crossref] [PubMed]
- Danaei G, Vander Hoom S, Lopez AD, et al. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005;366:1784-93. [Crossref] [PubMed]
- Carranza FA. Epidemiology of gingival and periodontal diseases. In: Carranza FA, Newman MG, Takei H. editors. Textbook of Carranza's clinical periodontology. St Louis: Missouri Saunders, Elsevier, 2006:86-91.
- Nyren O, Adami HO. Stomach cancer. In: Adami HO, Hunter D, Trichopoulos D. editors. Textbook of cancer epidemiology. New York: Oxford University Press, 2002:162-87.
- Nomura A. Stomach cancer. In: Schottenfeld D, Fraumeni JF. editors. Cancer epidemiology and prevention. New York: Oxford University Press, 1996:707-24.
- Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med 2000;248:171-83. [Crossref] [PubMed]
- Pritchard DM, Crabtree JE. Helicobacter pylori and gastric cancer. Curr Opin Gastroenterol 2006;22:620-5. [Crossref] [PubMed]
- Ozdemir A, Mas MR, Sahin S, et al. Detection of Helicobacter pylori colonization in dental plaques and tongue scrapings of patients with chronic gastritis. Quintessence Int 2001;32:131-4. [PubMed]
- Kim N, Lim SH, Lee KH, et al. Helicobacter pylori in dental plaque and saliva. Korean J Intern Med 2000;15:187-94. [Crossref] [PubMed]
- Dye BA, Kruszon-Moran D, McQuillan G. The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in theUnited States. Am J Public Health 2002;92:1809-15. [Crossref] [PubMed]
- Umeda M, Kobayashi H, Takeuchi Y, et al. High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J Periodontol 2003;74:129-34. [Crossref] [PubMed]
- Riggio MP, Lennon A. Identification by PCR of Helicobacter pylori in subgingival plaque of adult periodontitis patients. J Med Microbiol 1999;48:317-22. [Crossref] [PubMed]
- Asikainen S, Chen C, Slots J. Absence of Helicobacter pylori in subgingival samples determined by polymerase chain reaction. Oral Microbiol Immunol 1994;9:318-20. [Crossref] [PubMed]
- Oshowo A, Tunio M, Gillam D, et al. Oral colonization is unlikely to play an important role in Helicobacter pylori infection. Br J Surg 1998;85:850-2. [Crossref] [PubMed]
- Astrøm AN, Rise J. Socio-economic differences in patterns of health and oral health behaviour in 25-yearold Norwegians. Clin Oral Investig 2001;5:122-8. [Crossref] [PubMed]
- Thomson WM, Locker D. Dental neglect and dental health among 26-year-olds in the Dunedin Multidisciplinary Health and Development Study. Community Dent Oral Epidemiol 2000;28:414-8. [Crossref] [PubMed]
- Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol 2000;71:743-51. [Crossref] [PubMed]
- Bergstrom J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol 2000;71:1338-47. [PubMed]
- Hiraki A, Matsuo K, Suzuki T, et al. Teeth loss and risk of cancer at 14 com mon sites in Japanese. Cancer Epidemiol Biomarkers Prev 2008;17:1222-7. [Crossref] [PubMed]
- Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent 2010;38:83-95. [Crossref] [PubMed]
- Meyer MS, Joshipura K, Giovannucci E, et al. A review of the relationship between tooth loss, periodontal disease,and cancer. Cancer Causes Control 2008;19:895-907. [Crossref] [PubMed]
- Salaspuro MP. Acetaldehyde, microbes, and cancer of the digestive tract. Crit Rev Clin Lab Sci 2003;40:183-208. [Crossref] [PubMed]
- Homann N, Tillonen J, Rintamaki H, et al. Poor dental status increases acetaldehyde production from ethanol in saliva: a possible link to increased oral cancer risk among heavy drinkers. Oral Oncol 2001;37:153-8. [Crossref] [PubMed]
- Shapiro KB, Hotchkiss JH, Roe DA. Quantitative relationship between oral nitrate- reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chem Toxicol 1991;29:751-5. [Crossref] [PubMed]
- Hung HC, Colditz G, Joshipura KJ. The association between tooth loss and the self- reported intake of selected CVD-related nutrients and foods among US women. Community Dent Oral Epidemiol 2005;33:167-73. [Crossref] [PubMed]
- Greenstein G, Caton J, Polson AM. Histologic characteristics associated with bleeding after probing and visual signs of inflammation. J Periodontol 1981;52:420-5. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol 2005;76:2106-15. [Crossref] [PubMed]
- Moss SF, Blaser MA. Mechanisms of disease: inflammation and the origins of cancer. Nat Clin Pract Oncol 2005;2:90-7; quiz 1 p following 113.
- Fives-Taylor PM, Meyer DH, Mintz KP, et al. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000 1999;20:136-67. [Crossref] [PubMed]
- Zadeh HH, Nichols FC, Miyasaki KT. The role of the cell-mediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontol 2000 1999;20:239-88. [Crossref] [PubMed]
- Dye BA, Choudhary K, Shea S, et al. Serum antibodies to periodontal pathogens and markers of systemic inflammation. J Clin Periodontol 2005;32:1189-99. [Crossref] [PubMed]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007;117:1175-83. [Crossref] [PubMed]
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases Periodontol 2000 2004;34:9-21. [Crossref] [PubMed]
- Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol 2013;84:S8-19. [Crossref] [PubMed]
Cite this article as: Chrysanthakopoulos NA, Reppas SA, Oikonomou AA. Association of periodontal disease indices with risk of gastric adenocarcinoma. Ann Res Hosp 2017;1:4.


