Management of trauma-induced coagulopathy (TIC): a synopsis of the updated European trauma guideline
Introduction
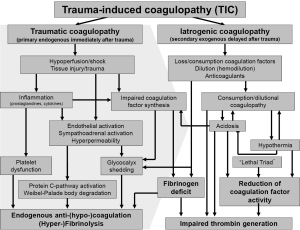
Uncontrolled hemorrhage and trauma-induced coagulopathy (TIC) are still the major causes for preventable death after trauma (1,2). Approximately one out of four severely injured trauma patients admitted to the hospital is bleeding with variable degrees of laboratory coagulopathy (3). Meanwhile, TIC is recognized as an own clinical entity with substantial impact on outcome and survival after trauma (4). There has been speculation about the potential mechanisms underlying TIC but much of the data continues to be rather correlative than causative with robust links still lacking (5). The current understanding of TIC is summarized in Figure 1. However, only little data has been reported linking the laboratory-based abnormalities with true clinically evident coagulopathic bleeding and therefore, TIC continues to be a significant diagnostic and therapeutic challenge.
Early detection and aggressive management of TIC have been associated with improved outcomes and there is need for the implementation of evidence-based local protocols and algorithms including clinical quality and safety management systems together with parameters to assess key measures of bleeding control and outcome. Recent surveys confirm a substantial diversity and heterogeneity in the clinical diagnosis and management of acute trauma hemorrhage and TIC across trauma centers in Europe and abroad (6). The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition has recently been updated and presents a total of 39 evidence-based recommendations for improved care for bleeding trauma patients and TIC (7). Thirteen out of these 39 recommendations are related to surgical measures and interventions, 24/39 are related to measures to control and stabalize hemostasis, and the remaing two recommendations refer to infrastructure, quality control and safety. Major aspects of the European trauma guideline have also been adopted to national and local guidelines in Europe (4). The overall therapeutic aim, if possible, is to rapidly detect and to stopp the bleeding.
GRADE of recommendations by the European trauma guideline
The recommendations given by the European trauma guideline are graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (8). The number associated with the recommendation reflects the strength of the recommendation being “GRADE 1” considered as the authors group’s strong recommendation and being “GRADE 2” considered as the authors group’s weak recommendation/suggestion; the following letter reflects the quality of the scientific evidence ranging from A (high quality evidence) to C (low quality evidence).
Prehospital management of the bleeding trauma patient
Relatively few hospitals offer the entire range of care for the multiple injured and bleeding trauma patient and a number of health care systems have thus developed and implemented trauma networks and systems. The overall aim is to optimize patient flow and to deliver appropriate and adequate care to the bleeding trauma patient as quick as possible. This is reflected by the European trauma guideline as the time elapsed between injury and (surgical) bleeding control should be minimised (GRADE 1A). Despite low evidence, there is consensus that organizing a “trauma system” is associated with a 15% reduction in trauma mortality with cases of “preventable deaths” reduced by half (9). In the context of time-sensitive pathologies such as traumatic brain injury (TBI), spinal cord injury, severe burns and hemorrhage, direct admission to a designated trauma center offering the maximum rage of care has been linked to improved survival (10). Therefore, the European trauma guideline suggests that the severely injured patient should be transported directly to an appropriate trauma center (GRADE 1B). In the translation of military experience gained in the field into the civilian arena, the early use of tourniquets and pelvic binders to stopp life-threatening bleeding from open extremity injuries and internally from pelvic fractures is now highly recommended. This recommendation has been lifted from a GRADE 1C to a GRADE 1B recommendation and, in Germany, all emergency vehicles are currently being equipped with these potentially life-saving devices.
Initial resuscitation of the bleeding trauma patient
In severely injured and hypotensive trauma patients, volume replacement should be initiated, at a reduced level if there is uncontrollable bleeding, in order to keep the circulation stable at target blood pressure and not excerbate the bleeding until the bleeding can be controlled (GRADE 1B). The European trauma guideline suggests for adult trauma patients with active bleeding to conduct “permissive hypotension” with target mean arterial pressure (MAP) 65 mmHg and/or with target systolic blood pressure (SBP) 80–90 mmHg until major bleeding control has been achieved (GRADE 1C). The fluid of choice in the hypotensive bleeding trauma patient is isotonic crystalloid solution (GRADE 1A); the use of colloids be restricted due to their adverse effects on hemostatis (GRADE 2C). Specific recommendations apply for patients with TBI.
In-hospital management
Clinical assessment of the bleeding trauma patient
The extent of trauma hemorrhage should be clinically assessed by using a combination of patient physiology, anatomical injury, mechanism of injury and the individual’s response to initial resuscitation as outlined in the “Advanced Trauma Life Support”-protocol (GRADE 1C) (11). This concept suggests four classes of hypovolemic shock based upon initial presentation which trigger specific strategies for initial fluid resuscitation. Recently, the validity of this classification has been questionned to that extent as when the given criteria were applied to trauma patients captured into the German TraumaRegistry® (TR-DGU®) database only a low percentage of patients could be adequately classified (12). Early and repeated imaging such as computed tomography (CT) and ultrasound, e.g., in the context of focused assessment sonography in trauma (FAST), is recommended to detect or exclude extravasal fluid (GRADE 1B). The number of CT scans including whole-body CT scans has seen an explosion since the landmark study by Huber-Wagner and colleagues from the TR-DGU® was published indicating a survival benefit in severely injured patients along with the integration of whole-body CT into early trauma care (13). Currently, three out of four trauma patients receive CT diagnostics (either cranial CT or whole-body CT) within mean 23±17 minutes after hospital admission and over four out of five patients receive FAST ultrasound diagnostics within mean 6±10 minutes after hospital admission in trauma centers affiliated with the TR-DGU®.
Laboratory parameters
Monitoring and measures to support coagulation function should be initiated immediately after Emergency Room admission of the bleeding trauma patient. This recommendation has received an augmentation from initially GRADE 1C to GRADE 1B in the updated guideline. Low initial hemoglobin (Hb) is considered indicative for severe bleeding associated with coagulopathy and repeated Hb measurements are recommended as an initial value within in reference ranges may mask the bleeding (GRADE 1B). Laboratory parameters to assess and monitor volume deficits and shock include lactate, ScvO2, hematocrit and base excess (BE). The European trauma guideline advocates repeated and independent measurements of BE and/or lactate as sensitive tests to assess adequate perfusion, to estimate and monitor the extent of bleeding and shock and to guide volume replacement therapy (GRADE 1B). A BE of less than −6 mEq/L is considered as an alert criteria for TIC being strongly associated with hypofibrinogenemia. The reliability of lactate may be lower when trauma is coincidenced with alcohol consumption; in these cases BE may be a superior predictor. Recent data from the TR-DGU® showed that BE upon admission may be superior over the ATLS classification of hemovolemic shock in predicting the need for volume including transfusion and mortality (14).
Conventional coagulation assays (CCAs) and viscoelastic testing assays
The routine practice should include the early and repeated monitoring of coagulation, using either CCAs such as prothrombin time (PT), activated partial thromboplastin time (aPTT), platelet count and fibrinogen (GRADE 1A) and/or viscoelastic testing assays (GRADE 1C). As early variables of clot firmness detected via viscoelastic testing assays have been associated with outcome in bleeding trauma patients, a rapid and more complete monitoring of the individual’s coagulation profile including fibrinolysis may facilitate a more accurate targeting of therapy as compared to isolated CCAs. In addition, CCAs only monitor the initiation phase of the clotting process and only 4% of the overall thrombingeneration (15). Turn-around times for CCAs, even in advanced trauma centers, may be substantial as compard to results from viscoelastic testing assays and are likely to delay effective hemostatic treatment. Fibrinogen levels deteriorate before other routine coagulation parameters and massive transfusion in the early phase of severe trauma and low levels on admission have frequently been associated with poor outcome (16).
Surgical bleeding control
Patients with significant intrathoracic, intraabdominal or retroperitoneal bleeding and hemodynamic shock and instability with ongoing bleeding and coagulopathy need to undergo urgent and immediate surgical intervention (GRADE 1A) according to “Damage control”-surgery principles (GRADE 1B) (17). Meanwhile, the procedure of emergency thoracotomy has clear and accepted indications, while procedures such as REBOA (Resuscitative Endovacular Balloon Occlusion of the Aorta) need further evaluation. Interventional radiology may be useful in pelvic bleeding but requires corresponding infrastructure.
Initial coagulation management including massive transfusion
In the acute phase, the clinical management of severe and bleeding trauma patients usually follows the “Damage Control Resuscitation” (DCR)-concept which advocates the empiric administration of blood products in predefined and fixed ratios (18). However, the optimum ratio is still under debate, no universal standard for the composition of these transfusion packages has yet been established and storage time may considerably affect the hemostatic competence of these products. Recent evidence suggests that this approach may also not be adequate to correct hypoperfusion or coagulopathy during the acute phase of trauma hemorrhage. As an alternative, several European but also a few US trauma centers have instituted the concept of “Goal-directed Coagulation Therapy” (GDCT) based upon results obtained from early point-of-care (POC) viscoelastic testing assays (19). Viscoelastic testing assays provide rapid information about the underlying deficiencies with particular focus on the different aspects of hemostasis such as initiation, dynamics and sustainability of clotting thus allowing targeted coagulation monitoring and therapy according to the individual’s needs (Figure 2). A recently updated Corchrane-review provided, apart from the know reductions in transfusion requirement, for the first time, a survival benefit with the use of viscoelastic testing assays in adults or children with bleeding (20).

Massive transfusion
The European trauma guideline currently advocates one of the two following strategies for the initial management of patients with bleeding and (expected risk of) massive transfusion: (I) plasma [fresh frozen plasma (FFP) or pathogen-inactivated plasma] in a plasma:packed red blood cell (pRBC) ratio of at least 1:2 (GRADE 1B); or (II) fibrinogen concentrate and pRBC according to the individual Hb level (GRADE 1C). Further resuscitation measures should be continued using a goal-directed strategy guided by CCAs and/or viscoelastic testing assays (GRADE 1C).
Plasma-based versus factor-concentrate based strategies
In the absence of massive transfusion and if a plasma-based coagulation resuscitation strategy is used, the European trauma guideline recommends plasma (FFP or pathogen-inactivated plasma) be administered to maintain PT and aPTT <1.5 times the normal control (GRADE 1C). It is emphasized that plasma transfusion be avoided in patients without substantial bleeding (GRADE 1B). If a factor concentrate-based strategy is executed, the treatment with fibrinogen concentrate (or cryoprecipitate) is advocated if significant bleeding is accompanied by viscoelastic signs of a functional fibrinogen deficit or a fibrinogen level <1.5–2.0 g/L (GRADE 1C). Once fibrinogen levels have been corrected and provided that fibrinogen levels are within the reference ranges but coagulation initiation is still delayed based on evidence from viscoelastic monitoring, the European trauma guideline suggests the administration of prothrombin complex concentrates (PCC) or plasma in the bleeding trauma patient (GRADE 2C). Repeated factor doses should be guided by viscoelastic monitoring and laboratory assessment of fibrinogen levels (GRADE 2C). A target level for Hb of 7–9 g/dL (GRADE 1C) is suggested while platelet concentrations should be kept >50×109/L (GRADE 1C); in patients with TBI and/or ongoing bleeding >100×109/L (GRADE 2C).
Viscoelastic testing assays (ROTEM®) to guide hemostatic therapies
Thromboelastometry (ROTEM®) is increasingly being used to diagnose, monitor and guide treatment strategies in trauma hemorrhage but currently, no uniformly accepted guidelines exist for how this technology should be integrated into clinical care. In September 2014, an international multidisciplinary group of leaders mostly from Europe but also from the Americas in the field of trauma coagulopathy and resuscitation was assembled to agree upon viscoelastic thresholds for triggering the initiation of specific treatments including fibrinogen, platelets, plasma, and PCCs in the acutely bleeding trauma patient. The consensus corresponds to a S2k guideline according to the system of the Association of the Scientific Medical Societies in Germany (AWMF) and which informs, for the first time, on specific triggers for clinical descision making (21).
Tranexamic acid (TXA)
Fibrinolysis has been identified as an integral component to the pathogenesis of TIC (22) and based upon CRASH-2, the use of the synthetic lysine analogue TXA as early as possible in the trauma patient who is bleeding or at risk of significant hemorrhage is highly recommended (23). Despite substantial methodological problems associated with this study, the recommendation in favor of the early administration of TXA is one of the very few GRADE 1A recommendations of the European trauma guideline. TXA should not be given after three hours of injury due to potentially adverse effects (GRADE 1B); the prehospital administration of TXA in bleeding trauma patients en route to the hospital may be considered (GRADE 2C). A number of emergency medical services (EMS) around the globe have yet implemented TXA into their local prehospital treatment algorithms.
Recombinant activated coagulation factor VII (rFVIIa) and desmopressin (DDAVP)
Due to insufficient high-level evidence and failure in large clinical trials, recombinant activated coagulation factor VII (rFVIIa) is currently recommended only for off-label use if major bleeding and TIC persist despite all other attempts to control the bleeding and best practice use of conventional hemostatic measures (GRADE 2C). It needs to be emphasized that rFVIIa acts on the individual’s own coagulation system sufficient numbers of platelets and fibrinogen concentrations provided. The European trauma guideline argues against the rountine use of desmopressin (DDAVP) in the bleeding trauma patient (GRADE 2C).
Environmental conditions
Even small reductions in pH and temperature result in reduced coagulation emzyme kinetics. The European trauma guideline recommends to avoid hypoxemia and acidosis rather than to correct (GRADE 1A) as experimental simple correction of the arterial pH with bicarbonate was not sufficient for reversal of coagulopathy due to acidosis (24). Likewise, the early application of measures to reduce heat loss and warm the hypothermic patient in order to achieve and maintain normothermia is recommended (GRADE 1C). Ionised calcium levels should be monitored and maintained within the normal reference ranges during (massive) transfusion (GRADE 1C).
Quality and safety management systems
The European trauma guideline strongly suggests that institutions that admit and treat severely injured and bleeding trauma patients should develop and provide a local and evidence-based treatment algorithm (GRADE 1B) while simultaneously take measures to control the adherence to these algorithms in the context of quality control and safety (GRADE 1C).
Acknowledgements
None.
Footnote
Conflicts of Interest: Marc Maegele has received honoraria from Astra Zeneca, CSL Behring, LFB Biomedicaments France, TEM International.
References
- Evans JA, van Wessem KJ, McDougall D, et al. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg 2010;34:158-63. [Crossref] [PubMed]
- Schoeneberg C, Schilling M, Hussmann B, et al. Preventable and potentially preventable deaths in severely injured patients: a retrospective analysis including patterns of errors. Eur J Trauma Emerg Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury 2007;38:298-304. [Crossref] [PubMed]
- S3 Guideline on Treatment of Patients with Severe and Multiple Injuries, English Version of the German Guideline S3 Leitlinie Polytrauma/Schwerverletzten-Behandlung AWMF Register-Nr. 012/019. Accessed 29 September 2016. Available online: htmlhttp://www.awmf.org.leitlinien/II/012-019.
- Chang R, Cardenas JC, Wade CE, et al. Advances in the understanding of trauma-induced coagulopathy. Blood 2016;128:1043-9. [Crossref] [PubMed]
- Schäfer N, Driessen A, Fröhlich M, et al. Diversity in clinical management and protocols for the treatment of major bleeding trauma patients across European level I Trauma Centres. Scand J Trauma Resusc Emerg Med 2015;23:74. [Crossref] [PubMed]
- Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care 2016;20:100.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest 2006;129:174-81. [Crossref] [PubMed]
- Celso B, Tepas J, Langland-Orban B, et al. A systematic review and meta-analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma 2006;60:371-8; discussion 378. [Crossref] [PubMed]
- Härtl R, Gerber LM, Iacono L, et al. Direct transport within an organized state trauma system reduces mortality in patients with severe traumatic brain injury. J Trauma 2006;60:1250-6; discussion 1256. [Crossref] [PubMed]
- American College of Surgeons Committee on Trauma. ATLS Student Manual 9th Edition. Chicago, IL: American College of Surgeons, 2012.
- Mutschler M, Nienaber U, Brockamp T, et al. A critical reappraisal of the ATLS classification of hypovolaemic shock: does it really reflect clinical reality? Resuscitation 2013;84:309-13. [Crossref] [PubMed]
- Huber-Wagner S, Lefering R, Qvick LM, et al. Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet 2009;373:1455-61. [Crossref] [PubMed]
- Mutschler M, Nienaber U, Brockamp T, et al. Renaissance of base deficit for the initial assessment of trauma patients: a base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the TraumaRegister DGU®. Crit Care 2013;17:R42. [Crossref] [PubMed]
- Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol 2003;23:17-25. [Crossref] [PubMed]
- Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012;10:1342-51. [Crossref] [PubMed]
- Ball CG. Damage control surgery. Curr Opin Crit Care 2015;21:538-43. [Crossref] [PubMed]
- Briggs A, Askari R. Damage control resuscitation. Int J Surg 2016;33:218-21. [Crossref] [PubMed]
- Schöchl H, Maegele M, Voelckel W. Fixed ratio versus goal-directed therapy in trauma. Curr Opin Anaesthesiol 2016;29:234-44. [Crossref] [PubMed]
- Wikkelsø A, Wetterslev J, Møller AM, et al. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev 2016.CD007871. [PubMed]
- Inaba K, Rizoli S, Veigas PV, et al. 2014 Consensus conference on viscoelastic test-based transfusion guidelines for early trauma resuscitation: Report of the panel. J Trauma Acute Care Surg 2015;78:1220-9. [Crossref] [PubMed]
- Kashuk JL, Moore EE, Sawyer M, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg 2010;252:434-42; discussion 443-4. [PubMed]
- CRASH-2 trial collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32.
- Darlington DN, Kheirabadi BS, Delgado AV, et al. Coagulation changes to systemic acidosis and bicarbonate correction in swine. J Trauma 2011;71:1271-7. [Crossref] [PubMed]
Cite this article as: Maegele M. Management of trauma-induced coagulopathy (TIC): a synopsis of the updated European trauma guideline. Ann Res Hosp 2017;1:6.