Primary angiosarcoma of the breast in a seventy-year-old female: a rare case report
Introduction
Primary angiosarcoma (AS) is a rare type of breast malignancy lesion accounting for only 0.04% of all malignant breast lesions (1). AS is a malignant breast lesion that arises from stromal tissue. It is extremely rare and accounts for <1% of all malignant breast tumors (1). Statistically <10% of ASs occur in the breast (2).
AS can be classified into primary and secondary. Primary AS develops in patients who do not have a history of breast cancer treatment. Secondary AS on the contrary develops in women with a history of breast cancer treatment.
Primary AS occurs in young woman in 20–40 years while secondary AS will tend to develop in woman over 40 years & can develop 5–10 years after radiation. About 17% patients develop AS after radiation for treatment of breast carcinoma.
Case presentation
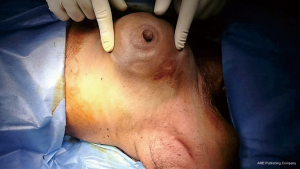
A 70-year-old female came to our OPD with complains of a progressively increasing left breast lump. She was previously treated in some private hospital as a case of carcinoma breast diagnosed on FNAC. She received five cycles of chemotherapy (FEC) without any response. After that she consulted to our OPD. On examination there was a well-defined, mobile, nontender and firm lump of 8 cm × 10 cm size (Figure 1). Skin was not involved and there was no palpable axillary lymphadenopathy. Sonomammography revealed a thick walled cystic mass filled with turbid fluid in left breast.
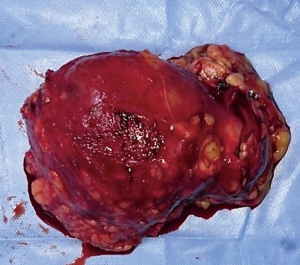
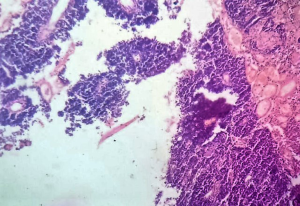
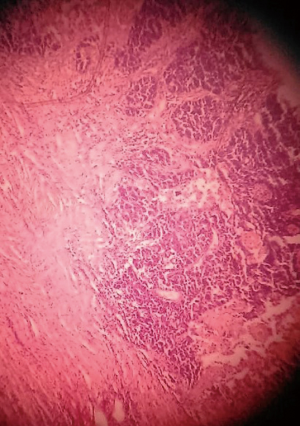
We sent a trucut biopsy of the lesion that revealed an AS. After metastatic workup we took the patient for surgery and a wide local excision (WLE) was done (Figure 2). On sectioning the specimen, dark brown fluid came out of a cystic cavity mass. The inside of the cavity was also filled with soft friable polypoidal tissue (Figure 3). Final histopathological examination (HPE) was reported as AS breast with clear margins (Figures 4,5) further confirmed by IHC which was positive for CD34 and CD31.
Post operatively at discharge, the Patient was referred for radiotherapy. At two months follow up the patient was free of any evidence of recurrence.
Discussion
AS of the breast is most common sarcoma of the breast but is still relatively rare. AS almost occurs almost exclusively in the female breast, with only five cases of male breast AS reported in the literature (2).
Primary AS of the breast occurs around third to fourth decade of life (3). With 6–12% of the cases occurring during pregnancy (4,5). In our case the age of the patient is 70 years which is unusual.
AS normally present as a painless rapidly growing palpable mass (6,7). Few patients complain of pain and tenderness and 2% of patients may present with diffuse enlargement of the breast. Sometimes bluish red discoloration of the overlying skin may be noted (8). Nipple retraction, discharge, and axillary lymphadenopathy are generally absent. Bilateral tumors are rare but may be seen specially post menopausal women (1).
In most case series tumor was >4 cm in diameter, (in our case 8 cm × 10 cm). Patients with tumor <4 cm at presentation had better survival (8). Some reports suggested that tumor size does not correlate with survival (9).
Preoperative diagnosis of AS breast is difficult by FNAC (in our case it was falsely reported as ductal carcinoma). Chen et al. (4) reported that false negative rate of FNAB is 37%. True cut biopsy or incisional biopsy is better for diagnosis.
The differential diagnosis of AS includes cystosarcoma phyllodes, benign hemangioma, stromal sarcoma, metaplastic carcinoma, squamous cell carcinoma with sarcomatoid features, myoepithelioma, fibrosarcoma, fibromatosis, liposarcoma and reactive spindle cell proliferative lesions (4).
Ultrastructural examination shows vascular nature of AS and exhibit existence of Weibel-Palade bodies and pinocytic vesicles. Immunohistochemistry for factor VIII related antigen is helpful for the diagnosis of AS of the breast (10). Antibodies directed against CD34 and CD31 identify nearly all AS including the poorly differentiated variant.
Chen et al. (4) overviewed the common sites of metastasis of primary AS of the breast as lung, skin, bone, liver, brain and ovary in order of frequency. Bones, skin and the contralateral breast metastasis are not commonly seen in other type of sarcoma (6). Axillary lymph node metastases are rare (9,11,12).
There is no gold standard treatment for AS. Due to infrequent incidence of disease there are no randomized trials comparing WLE with mastectomy. WLE is used for small lesions where there is high probability of achieving negative margins. Total mastectomy alone or with axillary node dissection is the preferred method of treatment. Mastectomy is more likely to result in an R0 resection (with a margin of >2 cm) (13). Deep margin is most commonly positive in an incomplete excision suggesting that more aggressive excision including muscle may be required. The necessity of axillary dissection is debatable at present as nodal involvement is uncommon in AS. In a series, reported nodal involvement is <10% (14).
Chemotherapy—some studies suggest that treatment with anthracycline based chemotherapy is useful in improving both disease free survival (DFS) and overall survival (OS) (15). A meta-analysis of patients treated with doxorubicin and a randomized trial of epirubicin plus ifosfamide demonstrated longer DFS and OS (5,16). In two studies, adjuvant chemotherapy had no effect on recurrence free survival or OS (17,18).
A retrospective analysis of 41 patients, published in 2,011 among patients with metastatic ASs from different primary tumor sites demonstrating an improvement in OS from 10.4 to 23.7 months with taxane based regimens compared to non-taxane based adjuvant treatment (19).
Radiotherapy—radiation treatment as an adjuvant setting is used with the intent of improving locoregional control after surgical excision and for survival. In one review of ten series of patients with ASs of the breast 35% underwent adjuvant radiation (15). Treatment was based on tumor characteristics and the type of surgical treatment given (17). Even after radical surgery i.e., mastectomy, radiotherapy has been thought to be beneficial for patient with microscopically positive margins (20). However in one series of 14 out of 63 patients treated with radiation either alone or in combination with chemotherapy, no significant difference in local control or survival was noticed (17). In two series, a benefit to the 5–10 years’ recurrence free survival, DFS and OS was seen following radiotherapy (5,18).
According to Rosen’s study, the 5 years survival rate for low grade sarcomas can be 76% while 70% for intermediate grade, whereas high grade sarcoma has worst survival of approximately 15% (5).
Conclusions
Our case report of this rare primary breast AS is exclusive as of the very few reported cases so far, this is the only one presenting in a woman in the seventh decade of her life and is exceptionally oversized for a primary AS at the time of presentation.
Whenever under clinical suspicion, a tissue biopsy is the recommended gold standard investigation since fine needle aspiration (FNA) has been reported to yield a high false negative result.
Since there are no established set goals for treating breast AS till date, surgical axillary clearance along with WLE/mastectomy still remains controversial since the axillary LN metastasis is very rare.
So far the Trials done on the role of chemo radio therapy in treating breast AS have revealed controversial results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bhosale SJ, Kshirsagar AY, Patil MV, et al. Primary angiosarcoma of breast: A case report. Primary angiosarcoma of breast: A case report. Int J Surg Case Rep 2013;4:362-4. [Crossref] [PubMed]
- Halls S. Breast Angiosarcoma. Moose and Doc Breast Cancer. October 5, 2016.
- Hacking EA, Tiltman AJ, Dent DM. Angiosarcoma of the breast. Clin Oncol 1984;10:177-80. [PubMed]
- Chen KT, Kirkegaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer 1980;46:368-71. [Crossref] [PubMed]
- Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma. The prognostic significance of tumor differentiation. Cancer 1988;62:2145-51. [Crossref] [PubMed]
- Johnson CM, Garguilo GA. Angiosarcoma of the breast: A case report and literature review. Curr Surg 2002;59:490-4. [Crossref] [PubMed]
- Georgiannos SN, Sheaff M. Angiosarcoma of the breast: a 30 year perspective with an optimistic outlook. Br J Plast Surg 2003;56:129-34. [Crossref] [PubMed]
- Donnell RM, Rosen PP, Lieberman PH, et al. Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol 1981;5:629-42. [Crossref] [PubMed]
- Ohta M, Tokuda Y, Kuge S, et al. A case of angiosarcoma of the breast. Jpn J Clin Oncol 1997;27:91-4. [Crossref] [PubMed]
- Patel T, Ohri SK, Sundaresan M, et al. Metastatic angiosarcoma of the ovary. Eur J Surg Oncol 1991;17:295-9. [PubMed]
- Edwards AT, Kellett HS. Haemangiosarcoma of breast. J Pathol Bacteriol 1968;95:457-9. [Crossref] [PubMed]
- Seinen JM, Styring E, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol 2012;19:2700-6. [Crossref] [PubMed]
- Kaklamanos IG, Birbas K, Syrigos KN, et al. Breast angiosarcoma that is not related to radiation exposure: a comprehensive review of the literature. Surg Today 2011;41:163-8. [Crossref] [PubMed]
- Fodor J, Orosz Z, Szabó E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol 2006;54:499-504. [Crossref] [PubMed]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573-81. [Crossref] [PubMed]
- Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 2001;19:1238-47. [PubMed]
- Sher T, Hennessy BT, Valero V, et al. Primary angiosarcomas of the breast. Cancer 2007;110:173-8. [Crossref] [PubMed]
- Hirata T, Yonemori K, Ando M, et al. Efficacy of taxane regimens in patients with metastatic angiosarcoma. Eur J Dermatol 2011;21:539-45. [PubMed]
- McGowan TS, Cummings BJ, O'Sullivan B, et al. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys 2000;46:383-90. [Crossref] [PubMed]
- Johnstone PA, Pierce LJ, Merino MJ, et al. Primary soft tissue sarcomas of the breast: local-regional control with post-operative radiotherapy. Int J Radiat Oncol Biol Phys 1993;27:671-5. [Crossref] [PubMed]
Cite this article as: Athar M, Singh S, Chaudhary AK, Sachan M, Khan L, Jamal S. Primary angiosarcoma of the breast in a seventy-year-old female: a rare case report. Ann Res Hosp 2017;1:7.