Diagnostic and therapeutic strategies for gastric cancer in the era of precision medicine
Introduction
Medical concepts are constantly evolving along with the advances in science and technology. Among them, the “precision medicine” and “precise surgery” are now sweeping the world, the “minimally invasive surgery” strives to limit the size of incisions, the “enhanced recovery after surgery” is designed to achieve the fastest recovery, and the “multidisciplinary cooperative team” emphasizes the important of team work in the treatment of tumors. These ideas will undoubtedly affect the clinical practice and have a profound impact on the diagnosis and treatment strategies of gastric cancer.
Evolution and existing concepts of medical concepts
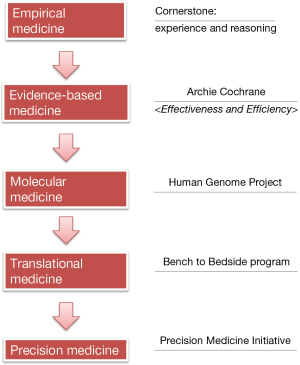
Diagnosis and treatment of diseases were mainly based on the doctors’ experiences decades ago (Figure 1). Every day the doctors made key decisions on disease treatment according to textbooks, experiences, and pathophysiological knowledge; this process is known as the empirical medicine, with experience and reasoning being its cornerstones. However, the potential defects of empirical medicine are obvious: it relies too much on the opinions of an individual expert and often lacks knowledge refreshment; as a result, it may neglect the new clinical findings that have substantial impacts on disease management.
In response to the potential defects of the empirical medicine, in 1972 the British epidemiologist Archie Cochrane published an influential book entitled Effectiveness and Efficiency: Random Reflections on Health Services, in which he proposed that all the medical professions should sort out the results of all randomized controlled trials and make corresponding evaluations; meanwhile, new findings should be continuously collected to update such evaluations, so as to provide reliable evidences for clinical practices, which is known as evidence-based medicine (EBM). This proposal has been widely accepted in the medical community and had a profound influence. EBM centers have been established throughout the world since then; in China, the first EBM center was established in West China Hospital in 1999, which was also the first EBM center in Asia. Currently all the EBM centers are named as Cochrane centers to commemorate his outstanding contribution to the EBM.
Along with the rapid development of science and technology, scientists have been able to explore the mechanisms of pathogenesis at the molecular level and medical research has entered a molecular era. The Human Genome Project was one of the most representative studies. It began in 1990 and costed nearly $3 billion USD to make a precise determination of all the base pairs that make up the human DNA, with an attempt to decipher the blueprint of life. Although the basic research at molecular level has achieved good results, one of the most serious problems facing the medical profession is: a large number of basic research has not been timely translated into productive forces to solve the problems encountered during clinical diagnosis and treatment, and many important diseases have not been effectively controlled or still lack effective prevention and control measures.
Therefore, the US National Institutes of Health (NIH) Clinical Center initiated the Bench-to-Bedside project in 1999. Bench and Bedside refer to the basic and clinical research, respectively. This project was designed to promote the translation from the findings of basic research to clinical interventions/treatments, which lead to the rise of translational medicine.
In January 2015, “precision medicine” was formally proposed in US President Obama’s State of the Union address. Since then, the term “precision medicine” quickly swept the world and opened a new horizon for medical science. In fact, early in 2011, the US National Research Council released a report titled Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease, in which it declared that biology had become a data-intensive science and the traditional taxonomy could not reflect the advances in molecular biology; to better understand the relationship between disease genotype and disease phenotype, a new biomedical research knowledge network should be established based on multidisciplinary efforts and a new system to classify disease should be created. Then, what is precision medicine? Dr. Francis Collins, Director of the NIH, published an article in the New England Journal of Medicine (NEJM) in January 2015 and argued that precision medicine was “prevention and treatment strategies that take individual variability into account” (1). The essence of precision medicine is to analyze, identify, verify, and apply the biological markers in large populations and for specific diseases by using omics (e.g., genomics and proteomics) and other sophisticated techniques, so as to accurately find the disease causes and therapeutic targets and perform accurate sub-classification of the different status and processes of a specific disease; by doing so, the clinicians may ultimately achieve the individualized accurate treatment of a disease in a patient and thus improve disease diagnosis, treatment, and prevention. In June 2015, the NEJM again arranged a discussion on the issues and perspectives of precision medicine in the decision-making consulting channel and pointed out that the intrinsic goal of the precision medicine is to improve the tailored clinical prognosis and to reduce the unnecessary side effects in non-responsive patients (2). It can therefore be concluded that the precision medicine is a medical model that utilizes a variety of cutting-edge technology and integrates multi-disciplinary knowledge to achieve the accurate classification of diseases and the individualized prevention and treatment of diseases, so as to ultimately improve the patients’ prognosis and minimize the treatment-related adverse reactions.
Despite the constant innovation and evolution of medical concepts, any new medical concept is to augment or enrich the old ones, rather than completely replace them. In addition to precision medicine, many other new medical concepts including precise surgery, minimally invasive surgery, enhanced recovery after surgery, and multidisciplinary cooperative team have also been proposed. All these medical concepts are governed by a common rule: a good doctor should practice should not only practice according to the standardized guidelines but also carry out tailored treatment according to the specific conditions of individual patients.
Diagnostic and therapeutic strategies for gastric cancer in the era of precision medicine
Disease classification, liquid biopsy, and outcome prediction
Precision medicine helps us to re-classify the diseases, achieve real-time liquid biopsy, and predict disease outcomes. The US National Cancer Institute and the Human Genome Research Institute jointly launched the Cancer Genome Atlas (TCGA) in 2005, in which the researchers classified the genetic mutations in tumors by using high-throughput sequencing technology and biological information technology; the study was designed to determine the sequences of over 20 common tumors including lung adenocarcinoma, papillary kidney carcinoma, ductal carcinoma of the breast, colon cancer, pancreatic duct cancer, and liver cancer. As part of the research plan, gene sequencing of the gastric cancer had been completed, and the results were published in Nature in 2014 (3). Unlike the traditional classification of gastric cancer based on location, etiology, and/or histology, the article proposed a molecular classification dividing gastric cancer into four subtypes: tumours positive for Epstein-Barr virus, microsatellite unstable tumours, genomically stable tumours, and tumours with chromosomal instability. Disease typing at the molecular level may help us to develop drug treatment therapies and may even affect the surgical practices. A 5-cm resection margin is required in the surgery for gastric cancer to minimize the risk of microscopic positive margin (R1 resection) because R1 resection is associated with relapse and shorter survival (4). However, the relationship between R1 resection and recurrence/survival is stage-specific (5). According to the study of the U.S. Gastric Cancer Collaborative, in patients with stage I distal gastric adenocarcinoma, a 3-cm proximal margin (PM) was associated with the same improved OS as a 5.0-cm margin (6); for proximal gastric cancer, the length of PM was not associated with the local relapse and OS, and therefore a specific PM length was not required (7). Thus, for gastric cancer that is conventionally classified according to the locations, the surgical practices will be different. Since gastric cancer can be divided into four types according to molecular types, different types of molecules will certainly have different malignant biological behaviors; it is reasonable to speculate that different molecular types should also have different margin lengths to achieve precision.
Precision medicine has enriched our traditional diagnostic and therapeutic strategies for gastrointestinal tumors. For example, traditionally we rely on blood tumor markers for diagnosis of gastrointestinal tumors and on pathological biopsy of tumor tissues to detect the expression of a specific gene, so as to predict the prognosis and guide the treatment. Fortunately, now we have a new option, that is, liquid biopsy. Unlike tissue biopsy, liquid biopsy detects tumors by determining markers in body fluids. It is less invasive and allows multiple determinations and real-time monitoring. At present, the commonly used liquid biopsy markers include circulating tumor cells (CTC), circulating tumor DNA, and circulating non-coding RNAs.
Patients with advanced colorectal cancer have low 5-year survival rate, and the case-fatality rate can dramatically drop if early diagnosis and treatment can be achieved. According to a prospective multicenter study published in the Journal of Clinical Oncology in 2008, if the baseline number of CTCs was >3/7.5 mL in colorectal cancer patients, the OS and PFS were relatively low, along with poor treatment effectiveness; however, if the CTC level dropped after treatment (<3/7.5 mL), a better prognosis could be expected (8). Research has also demonstrated that CTC level is an independent prognostic factor for PFS and OS in patients with metastatic colorectal cancer and can provide more prognostic information before any imaging change occurs (8). As a result, the US FDA has approved the use of CTC as an auxiliary test in clinical laboratories.
Determining the DNA mutations in CTCs can also guide precision treatment. According to a research publish in Nature in 2012, KRAS mutations could be detected in the plasma samples of colorectal patients with secondary resistance to cetuximab before radiographic progression. Therefore, testing for ctDNA can help us to judge any drug resistance and guide the further treatment strategy (9).
The circulating non-coding RNAs in the blood can also assist the clinical diagnosis. We have explored the roles of circulating micro RNAs (miRNA) in the diagnosis of gastric cancer and found that miRNA-214 had relatively high diagnostic value (10). Recently, some Japanese authors have explored the diagnostic value of long non-coding RNA (LncRNA) and found that LncRNA H19 had significantly different expression profiles in gastric cancer patients and normal populations, and the area under ROC curve was 0.64 (11).
Precise surgery
Sentinel lymph node navigation surgery offers the possibility of precise surgical treatment. The sentinel lymph node is the first node to receive drainage from the primary tumor and is therefore at the highest risk of tumor metastasis. As seen in literature, the lymph node metastasis rate was 2–18% for stage T1 gastric cancer and 20% for stage T2 gastric cancer; over 90% of patients with early gastric cancer survived more than 5 years, in whom the majority of the resected lymph nodes were negative (12,13). Theoretically, if there is no sentinel lymph node metastasis, there is no potential for lymph node metastasis; as a result, the treatment strategies will also be adjusted accordingly. During the sentinel lymph node navigation surgery for T1–2 gastric cancer without lymph node metastasis and sized <4 cm, tracer is injected around the tumor, followed by lymph node biopsy to identify any metastasis. According to the results of sentinel lymph node biopsy, the corresponding treatment plan will be established to achieve the individualized precise surgical treatment (13).
Minimally invasive surgery
In addition to precision, “minimally invasive” has also long been pursued by surgeons. The concept of minimally invasive surgery was initially proposed by the British surgeon John E. A. Wickham in 1980s, who also established the first minimally invasive surgery center in the United States (14). After nearly 30 years, minimally invasive surgery has been widely used in thoracic surgery, general surgery, urology, and other relevant fields.
For gastric cancer, minimally invasive surgery has two forms: laparoscopic surgery and robotic surgery. According to the guidelines released by the Japanese Gastric Cancer Association in 2014, laparoscopic surgery for distal gastric gastric is indicated in stage I tumor only; for advanced gastric cancer, laparoscopic gastrectomy for distal gastric cancer and total gastrectomy can only used for research purpose. As demonstrated in a retrospective study from the CLASS research group in China (published in 2014), laparoscopic-assisted surgery for advanced gastric cancer is safe and technically feasible, with acceptable short-term oncological outcomes: the OS and DFS were 85% and 77% in patients with stage II gastric cancer and 60.5% and 59.3% in those with stage III gastric cancer (15). According to a phase III trial in China that compared the application of laparoscopic and open surgeries in the treatment of advanced gastric cancer, the short-term outcomes showed that D2 radical operation could be safely performed by experienced surgeons (16).
The emergence of the robot has further enriched the connotation of minimally invasive surgery and brought many benefits to both patients and surgeons. Robotic surgery, as another main practice of minimally invasive surgery, has been further studied in the field of gastric cancer. As demonstrated in a multicenter prospective clinical study published in the Annals of Surgery in 2015, there was no statistical difference in terms of the postoperative complications and case-fatality rate between the robot assisted surgery and laparoscopic assisted surgery for gastric cancer; also, the intraoperative blood loss, rate of conversion to open surgery, and hospital stay were also comparable between these two groups (17). Our team had also retrospectively compared the short-term outcomes of the robotic and laparoscopic gastric cancer surgeries and found that the short-term outcomes were similar between these two techniques (18). In July 2015, a Korean team reported that robotic-assisted radical surgery for distal gastric cancer could dissect more lymph nodes in station 2, especially when dissecting lymph nodes in stations proximal to the splenic artery (19). Thus, the robotic surgery has comparable oncological outcomes with laparoscopic surgery, and meanwhile it also has many advantages such as accurate operation, clear 3D visual field, reduced effect of hand tremor, and feasibility of operation in a small space. Thus, we believe that robotic-assisted minimally invasive surgery will further reduce surgical stress and promote postoperative recovery. However, the majority of the currently available studies were retrospective studies with relatively small sample sizes; therefore, more well-designed prospective trials are needed to further confirm the potential advantages of robotic surgery in such areas as lymph node dissection.
Enhanced recovery after surgery
In clinical settings, the goal of “minimizing damage and speeding up recovery” also promotes the advances in surgery; as a result, the concept of “Enhanced Recovery After Surgery” (ERAS) occurred. In 2001, Kehlet proposed the concept of fast-track surgery; in 2010, an Enhanced Recovery After Surgery (ERAS) Society was founded in Sweden. Since then, the term ERAS has been widely used. ERAS not only requires optimal surgical approach, fine operation, and sophisticated surgical techniques but also requests optimal perioperative treatment, so as to accelerate postoperative recovery, reduce complications, and shorten the length of hospital stay. The connotation of surgical procedures should be a continuum that is based on the surgical operation but meanwhile also includes the meticulous perioperative management.
In the treatment of tumors, the ERAS Society has developed corresponding surgical procedures based on the different requirements of each surgery. For instance, guidelines for perioperative care in elective colonic surgery, in pancreaticoduodenectomy, and in elective rectal/pelvic surgery were established in 2012 (20-22). In 2014, the ERAS also released the consensus guidelines for enhanced recovery after gastrectomy (23), in which preoperative management of malnutrition, reducing medical procedures such as the placement of nasogastric tube and/or abdominal drainage tube, and early postoperative feeding/artificial nutrition were strongly recommended. It was also strongly recommended to carry out systematic review/evaluation to ensure the compliance of patients (23). Among the minimally invasive techniques used in surgery, laparoscopic surgery for early gastric cancer was also a strong recommendation due to its high evidence level; howerver, this technique was weakly recommended for advanced gastric cancer and total gastrectomy due to low evidence level (23).
In 2012, the Yamada et al. explored the usefulness of ERAS after surgery protocol as compared with conventional perioperative care in gastric surgery and found that the ERAS group had relatively short operative time. In terms of short-term outcomes, the first days of oral intake, oral intake recovery, flatus, and defecation were significantly earlier in the ERAS group than in the conventional care group. Maximum pain evaluated on a visual analog scale and the number of additional analgesics on demand were also significantly less in the ERAS group. Finally, the postoperative body weight change was smaller in the ERAS group (24).
In June 2015, the ERAS group investigated the impact of different levels of compliance on postoperative complications and hospital stay after elective primary colorectal cancer resection and further explored the impact of each ERAS care measure on the short-term outcomes (25). A total of 2,352 patients from 13 centers in 6 countries were enrolled in this study. It was found that the minimally invasive technique (i.e., laparoscopic surgery) in ERAS shortened the hospital stay; increasing ERAS compliance was correlated with shorter hospital stay. Among factors that might affect the postoperative complications, the minimally invasive technique (i.e., laparoscopy) was also a protective factor (OR: 0.68; 95% CI: 0.62–0.74). Increasing ERAS compliance was also correlated with fewer postoperative complications. This analysis has demonstrated that increasing compliance with an ERAS program and the use of laparoscopic surgery is helpful to reduce postoperative complications, shorten hospital stay, and thus accelerate patient recovery (25).
Multidisciplinary collaboration team
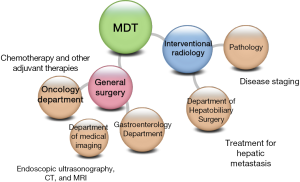
The treatment of cancer not only needs surgical operation but also requires the multidisciplinary collaboration among the departments of medical imaging, pathology, and oncology, so as to improve the quality of diagnosis and treatment and thus improve the prognosis (Figure 2).
In a multidisciplinary collaboration team, the oncologists shall establish reasonable preoperative and postoperative chemotherapy to improve the disease conditions. Chemotherapy is an important part of multidisciplinary treatment. However, partial resistance to chemotherapy has long been a major clinical problem. Therefore, if we were able to screen out drug-sensitive populations, as we decide the use of antibiotics based on the results of drug susceptibility testing, the treatment mode will undoubtedly meet the idea of precise medication. Patient-derived tumour xenografts may provide such a possibility. The patient-derived tumour xenografts were engrafted into nude mice, and the primary tumors grew in the nude mice were used for drug susceptibility testing. The results of drug susceptibility testing may guide the rational drug use (26). Chip analysis showed that the gene expressions of primary lesions in patients had good correlations with those in tumor xenografts in nude mice; thus, screening patient-sensitive drugs via xenograft tumors in nude mice is molecularly feasible (26).
In addition, developing molecular-targeted therapy is another task of a multidisciplinary collaboration team and also a key component of precision medicine. Four key studies have been carried out on the molecular-targeted therapy of gastric cancer: ToGA study, targeting HER2 (27); AVAGAST study, targeting vascular endothelial growth factor (VEGF) (28); EXPAND study, targeting epidermal growth factor receptor (EGFR) (29); REGARD study, targeting vascular endothelial growth factor receptor 2 (VEGFR2) (30). Among these studies, ToGA was quite successful, whereas both EXPAND and AVAGAST failed somehow, which might because the latter two studies did not include race, pathological type, molecular type, and targets in the study design. The future clinical studies should evaluate multiple molecular mutations based on histological findings and provide tailored treatment according to the results of gene typing. Meanwhile, molecular markers capable of predicting therapeutic efficacy should be screened out to identify drug-sensitive populations.
Opportunities and challenges
After the US President Obama’s Precision Medicine Initiative was unveiled in January 2015, government authorities and research institutions in China made a quick response. At the end of March, 2015, the National Health and Family Planning Commission (NHFPC) of China announced the list of first batch of pilot institutions that will apply the high-throughput gene sequencing technology in tumor diagnosis and treatment. The Ministry of Science and Technology also listed the precision medicine in the “13th Five-Year” national key R&D projects, and decided the funded projects in July 2016. Obviously, the Chinese government has paid special attention on precision medicine, and the corresponding supportive policies will certainly provide development opportunities for the implementation of precision medicine in China.
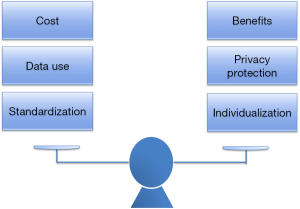
The era of precision medicine provides us with not only opportunities but also challenges. We need to balance the following three pairs of relationships (Figure 3): (A) costs and benefits. Practicing precision medicine will have high costs. For example, while CTC detection can assist diagnosis and prognosis, it is quite expensive. Also, the use of minimally invasive robot in ERAS is costly, not to mention the drugs used in the postoperative molecular targeted therapy. Thus, how to maximizing the benefit of the patients without increasing the economic burden of patients is one of the key issues to be addressed. (B) Data use and privacy protection: It is well known that the popularity of wearable devices provides the possibility to obtain the life parameters of patients in a real-time manner. For example, the information provided by a variety of sports bracelets and apple watches can facilitate decision-making on disease prevention and control and thus promote health. However, it is equally important to ensure that the patients’ health information and privacy will be well protected during the use of these data. (C) Precision medicine undoubtedly enriches our diagnosis and treatment strategies and enables us to provide tailored approaches to the patients. For instance, as mentioned above, the ERAS guidelines on surgeries for gastric cancer, pancreatic cancer, and duodenal cancer had their specific contents. However, standardized diagnosis and treatment is still required when providing individualized precise treatment. Precision and standardization shall be two essential principles of precision medicine.
Acknowledgements
Funding: National Key Research and Development Program: “Precision Medicine Research” (2016YFC0905302).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793-5. [Crossref] [PubMed]
- Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015;372:2229-34. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353-61. [Crossref] [PubMed]
- Bickenbach KA, Gonen M, Strong V, et al. Association of positive transection margins with gastric cancer survival and local recurrence. Ann Surg Oncol 2013;20:2663-8. [Crossref] [PubMed]
- Squires MH 3rd, Kooby DA, Poultsides GA, et al. Is it time to abandon the 5-cm margin rule during resection of distal gastric adenocarcinoma? A multi-institution study of the U.S. Gastric Cancer Collaborative. Ann Surg Oncol 2015;22:1243-51. [Crossref] [PubMed]
- Postlewait LM, Squires MH 3rd, Kooby DA, et al. The importance of the proximal resection margin distance for proximal gastric adenocarcinoma: A multi-institutional study of the US Gastric Cancer Collaborative. J Surg Oncol 2015;112:203-7. [Crossref] [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. [Crossref] [PubMed]
- Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012;486:532-6. [PubMed]
- Zhang KC, Xi HQ, Cui JX, et al. Hemolysis-free plasma miR-214 as novel biomarker of gastric cancer and is correlated with distant metastasis. Am J Cancer Res 2015;5:821-9. [PubMed]
- Arita T, Ichikawa D, Konishi H, et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res 2013;33:3185-93. [PubMed]
- Symeonidis D, Koukoulis G, Tepetes K. Sentinel node navigation surgery in gastric cancer: Current status. World J Gastrointest Surg 2014;6:88-93. [PubMed]
- Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol 2013;20:522-32. [Crossref] [PubMed]
- Fitzpatrick JM, Wickham JE. Minimally invasive surgery. Br J Surg 1990;77:721-2. [Crossref] [PubMed]
- Hu Y, Ying M, Huang C, et al. Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc 2014;28:2048-56. [Crossref] [PubMed]
- Hu Y, Huang C, Sun Y, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol 2016;34:1350-7. [Crossref] [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2016;263:103-9. [Crossref] [PubMed]
- Shen W, Xi H, Wei B, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc 2016;30:574-80. [Crossref] [PubMed]
- Kim YW, Reim D, Park JY, et al. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc 2016;30:1547-52. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:783-800. [Crossref] [PubMed]
- Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:817-30. [Crossref] [PubMed]
- Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:801-16. [Crossref] [PubMed]
- Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg 2014;101:1209-29. [Crossref] [PubMed]
- Yamada T, Hayashi T, Cho H, et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer 2012;15:34-41. [Crossref] [PubMed]
- ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg 2015;261:1153-9. [Crossref] [PubMed]
- Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 2012;9:338-50. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
Cite this article as: Zhang K, Huang X, Wei B, Wu X, Xi H, Peng Z, Cui J, Li J, Gao Y, Liang W, Hu C, Liu Y, Chen L. Diagnostic and therapeutic strategies for gastric cancer in the era of precision medicine. Ann Res Hosp 2017;1:8.