
MR findings of Creutzfeldt-Jakob disease: a rare entity
Introduction
Creutzfeldt-Jakob disease (CJD) is a spongiform encephalopathy commonly refer to as prion disease, presents mainly with progressive dementias, as well as a spectrum of neurologic symptoms and is ultimately fatal. Pathogenesis includes regular prion protein conversion to scrapie particles that are hypothesized to cause spongiform degeneration of the brain parenchyma signaling cell death (1). It is difficult to make a diagnosis alone by clinical examinations (2). However, MR imaging and especially diffusion-weighted imaging (DWI) has made it possible to diagnose this disease even in early stages (3). DWI features in early-stages are discrete and DWI is crucial for the diagnosis way before abnormal signal on T2WI and brain atrophy (4). In this case report, we will discuss the role of MR imaging in CJD with emphasis on the DWI and will discuss the common mimics and differentials.
Case presentation
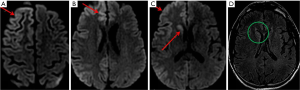
Our patient is a 45-year-old female with no significant past medical history who presented with altered mental status along with failure to concentrate for a period of one month. Patient presented to the emergency department with encephalopathy and exacerbating confusion. Physical examination showed that she was confused and non-communicative. Cognitive testing revealed problem in finger counting along with aphasia involving the expressions. Cogwheel type rigidity involving both upper and lower extremities was identified. There were loss of bilateral flexor plantar reflexes and primitive reflexes. Computed tomography (CT) scan was unremarkable for acute findings. Electroencephalography (EEG) revealed diffuse background slowing with findings showing higher involvement of right hemisphere. Subsequently, MR imaging of the brain was performed which showed abnormal hyperintensity within right caudate nucleus and putamina, the right occipital, temporal, and frontal gray matter on DWI (Figure 1A,B,C). These findings were seen in retrospect in some areas on FLAIR images (Figure 1D). There was no contrast enhancement after gadolinium administration.
Discussion
Various infections affect the brain and spine (5). CJD is a prion disease that causes progressive dementia and is invariably fatal. This results from the misfolding of normal cellular prion proteins into an abnormal conformation (6). The four classic types of CJD include sporadic, variant, familial, and iatrogenic, with sporadic CJD (sCJD) comprising up to 90% of the cases. The incubation period of CJD can range from several months to years. Symptoms include rapidly progressive dementia, ataxia, and myoclonus. Prompt diagnosis is essential to prevent human-to-human transmission. Currently, the only definite method of confirming the diagnosis of CJD is through biopsy. CSF analysis can also be performed, with the 14-3-3 protein used as a biomarker for the disease (7). However, this test remains controversial since it has a high sensitivity but low specificity for CJD. Once diagnosed, the prognosis of CJD is grim; 90% of patients die within 1 year of symptom onset. All victims of this disease will eventually die.
CT findings in CJD are most frequently normal, although in some cases CT can demonstrate rapidly progressive atrophic changes. As we know that in most of the brain and head and neck pathologies, MRI, especially DWI is the imaging modality of choice (8). Similarly, MRI has a critical role in the diagnosis of CJD (9).
One of the most commonly used diagnostic tools is EEG study, which in CJD is characterized by periodic bi- or triphasic synchronized wave complexes (PSWC). This pattern is typically observed in middle to late stages of the disease, and is therefore not as useful in early diagnosis (10). In addition, atypical EEG finding is only seen in approximately two-thirds of patients, yielding a sensitivity of 64% and specificity of 91% for this test (11). While the EEG of this patient showed diffuse cerebral dysfunction with more right hemisphere involvement, the typical PSMC associated with CJD was not observed.
On MR imaging, CJD shows high T2 signal in basal ganglia and cerebral cortex, as well as progressive brain atrophy. In early stages, T2-weighted images may be normal, making it burdensome to diagnose. However, DWI can show the areas of restricted diffusion in the cortex and in the basal ganglia or thalamus even before the electroencephalogram changes. DWI and FLAIR abnormalities in CJD are classically found in the bilateral thalamic pulvinar regions (12), which is mostly associated with the variant form of the disease. Thus, DWI is a crucial modality for prime diagnosis (13). As we know that anything which decreases the Brownian motion of the water molecules leads to restricted diffusion which is seen as increased signal on DWI and decreased signal on ADC map (14). The restricted diffusion in CJD is thought to be due to vacuolization of the neuropil leading to a restriction of water diffusion in the affected tissue, compared with that in normal tissue (15).
Since, CJD show diffusion restriction in the cerebral cortex, it is crucial to separate CJD from other conditions such as postictal state, venous hypertensive encephalopathy; herpes encephalitis, MELAS (which includes encephalopathy, mitochondrial myopathy, stroke like episodes and lactic acidosis). Absence of white matter involvement in CJD can be helpful in distinguishing CJD from other processes, as in the case with MELAS and infectious encephalopathies. Although white matter abnormalities are not visualized in MRI, histopathological studies revealed gliosis, microglial activation and vacuolization that suggest primary involvement of white matter in some cases (16). MRI changes associated with postictal state due to focal or generalized seizures are typically transient and reversible. On the other hand, the signal intensity can increase in CJD with disease progression up to the last stages of the disease, at which time observed MRI changes may decrease or even disappear presumably due to extensive neural death and atrophy (17).
Conclusions
Early diagnosis of CJD can be achieved with DWI MR imaging with high sensitivity before T2 or EEG abnormalities findings could corroborate. This is useful not only as an accurate diagnostic measure in CJD, early diagnosis can ensure proper safety measures to be taken to minimize its transmissibility. This method is also non-invasive and has been adapted as a diagnostic criterion for sporadic CJD. Together, along with clinical presentations, laboratory testing, and EEG study, this armamentarium would allow for more definitive diagnosis in absence of biopsy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Prusiner SB. Shattuck lecture: neurodegenerative diseases and prions. N Engl J Med 2001;344:1516-26. [Crossref] [PubMed]
- Barboriak DP, Provenzale JM, Boyko OB. MR diagnosis of Creutzfeldt-Jakob disease:significance of high signal intensity of the basal ganglia. AJR Am J Roentgenol 1994;162:137-40. [Crossref] [PubMed]
- Kumar Y, Drumsta D, Mangla M, et al. Toxins in Brain! Magnetic Resonance (MR) Imaging of Toxic Leukoencephalopathy- A Pictorial Essay. Pol J Radiol 2017;82:311-19. [Crossref] [PubMed]
- Bahn MM, Kido DK, Lin W, et al. Brain magnetic resonance diffusion abnormalities in Creutzfeldt-Jakob disease. Arch Neurol 1997;54:1411-15. [Crossref] [PubMed]
- Kumar Y, Gupta N, Chhabra A, et al. Magnetic resonance imaging of bacterial and tuberculous spondylodiscitis with associated complications and non-infectious spinal pathology mimicking infections:a pictorial review. BMC Musculoskelet Disord. 2017;18:244. [Crossref] [PubMed]
- Rosenbloom MH, Atri A. The evaluation of rapidly progressive dementia. Neurologist 2011;17:67-74. [Crossref] [PubMed]
- Chohan G, Pennington C, Mackenzie JM, et al. The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry 2010;81:1243-8. [Crossref] [PubMed]
- Bhatt N, Gupta N, Soni N, et al. Role of diffusion-weighted imaging in head and neck lesions: Pictorial review. Neuroradiol J 2017;30:356-69. [Crossref] [PubMed]
- Tian HJ, Zhang JT, Lang SY, et al. MRI sequence findings in sporadic Creutzfeldt-Jakob disease. J Clin Neurosci 2010;17:1378-80. [Crossref] [PubMed]
- Shin JW, Yim B, Oh SH, et al. Redefining periodic patterns on electroencephalograms of patients with sporadic Creutzfeldt-Jakob disease. Clin Neurophysiol 2017;128:756-62. [Crossref] [PubMed]
- Steinhoff BJ, Zerr I, Glatting M, et al. Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann Neurol 2004;56:702-8. [Crossref] [PubMed]
- Ukisu R, Kushihashi T, Kitanosono T, et al. Serial diffusion-weighted MRI of Creutzfeldt-Jakob disease. AJR Am J Roentgenol 2005;184:560-6. [Crossref] [PubMed]
- Tzeng BC, Chen CY, Lee CC, et al. Rapid spongiform degeneration of the cerebrum and cerebellum in Creutzfeldt-Jakob encephalitis: serial MR findings. AJNR Am J Neuroradiol 1997;18:583-6. [PubMed]
- Kumar Y, Wadhwa V, Phillips L, et al. MR imaging of skeletal muscle signal alterations:Systematic approach to evaluation. Eur J Radiol 2016;85:922-35. [Crossref] [PubMed]
- Ukisu R, Kushihashi T, Tanaka E, et al. Diffusion-weighted MR imaging of early-stage Creutzfeldt-Jakob disease: typical and atypical manifestations. Radiographics 2006;26 Suppl 1:S191-204. [Crossref] [PubMed]
- Caverzasi E, Mandelli ML, Dearmond SJ, et al. White matter involvement in sporadic Creutzfeldt-Jakob disease. Brain 2014;137:3339-54. [Crossref] [PubMed]
- Eisenmenger L, Porter MC, Carswell CJ, et al. Evolution of Diffusion-Weighted Magnetic Resonance Imaging Signal Abnormality in Sporadic Creutzfeldt-Jakob Disease, With Histopathological Correlation. JAMA Neurol 2016;73:76-84. [Crossref] [PubMed]
Cite this article as: Geng M, Sawhney H, Gupta S, Pittaro D. MR findings of Creutzfeldt-Jakob disease: a rare entity. Ann Res Hosp 2017;1:36.


