Diagnosis and treatment of tuberculosis: latest developments and future priorities
Background
Despite recent advances in diagnostics and treatment options, tuberculosis (TB) remains a substantial global health challenge. Worldwide, in 2015, there were 10.4 million new cases of TB and 1.8 million deaths attributable to the disease (1). Through intensified research, improvement in the provision of integrated patient care and implementation of sustainable health policies, the World Health Organisation (WHO) has set a target of reducing the TB incidence rate by 90% and the number of TB-related deaths by 95% by the year 2035, as outlined in its ‘End TB Strategy’ (2). The ultimate aim is to attain global eradication of TB.
However, to achieve these ambitions, several challenges pertaining to TB management will need to be overcome. In addition to addressing the burden of disease caused by drug-sensitive TB, it is important to tackle the increasing threat of multidrug-resistant (MDR) TB, defined as TB caused by M. tuberculosis isolates that are resistant to both rifampicin and isoniazid (1). Furthermore, a key component of TB eradication includes targeted identification and treatment of latent TB, an asymptomatic state in which the immune system of an individual has successfully contained the infection but a risk of converting to active TB disease remains should the immune response diminish or fail (3). Concurrent treatment of HIV-coinfection among TB patients is a further important issue.
This article provides an update on the clinical approach to managing drug-sensitive, drug-resistant and latent TB. The global epidemiology of the disease, clinical presentation and approaches to management are discussed. Future research and clinical priorities are considered.
Global epidemiology
Accurate estimates of TB incidence rely on robust national surveillance mechanisms that seek to address problems of under-reporting. TB incidence per country may be estimated using various approaches: case notification data may be combined with expert opinions on the likely degree of under-reporting and under-diagnosis; high-income countries may apply an adjustment factor to their reported notification number to account for under-reporting and under-diagnosis; incidence may be estimated using results from TB prevalence studies (1).
There was a 1.5% decrease in TB incidence globally between 2014 and 2015. In 2015, the country with the highest burden of TB disease was India, with an estimated total TB incidence of 2.84 million (uncertainty interval 1.47–4.65 million). Of the 10.4 million incident TB cases that year, 60% of cases were reported from the following six countries in decreasing order of incidence: India, Indonesia, China, Nigeria, Pakistan and South Africa. By region, 61% of cases were in Asia; 26% in Africa; 7% in the Eastern Mediterranean; 3% in Europe; and 3% in the Americas. HIV coinfection was reported in 11% of cases and was greatest in countries in southern Africa (1).
In the same year, there were 1.4 million deaths from TB among HIV-negative people (19 per 100,000 population), of which 84% occurred in Africa and Southeast Asia. There were a further 0.39 million deaths from TB among HIV-positive individuals. India and Nigeria accounted for 43% of the total number of TB deaths among HIV-negative and HIV-positive people combined. Overall, TB mortality rates have been falling in all continents and there has been a 34% reduction in the TB mortality rate between 2000 and 2015 (1).
MDR TB and rifampicin-resistant (RR) TB accounted for 3.9% of new TB cases and 21% of previously treated TB cases in 2015. MDR and RR TB accounted for 580,000 cases of incident TB cases and 250,000 deaths globally. China, India and Russia accounted for 45% of the global total MDR and RR TB case burden (1).
Diagnosis
Diagnosis of TB needs to take account of clinical history, microbiological results and radiological findings.
Clinical history and risk factors for TB
Clinical manifestations of TB disease depend upon the site of TB infection. Classically, pulmonary TB is characterised by a history of chronic cough, sputum production, haemoptysis, fever, night sweats and weight loss (4). The haematogenous spread of TB to multiple organs results in miliary TB.
Symptoms of extrapulmonary TB can be variable (5) and require a high degree of clinical suspicion:
- TB lymphadenitis is characterised by painless, progressive lymph node swelling (6). Cervical chain lymph nodes are the commonest site (7);
- Pleural TB may be asymptomatic but is often associated with the typical pulmonary symptoms of TB, including pleuritic chest pain (8);
- Central nervous system TB may manifest in the form of TB meningitis, tuberculomas or TB brain abscesses (9). TB meningitis usually presents with classical symptoms of meningitis, including headache, fever, meningism and focal neurological deficits (10);
- Skeletal TB includes: spinal TB (Pott’s disease), which is characterised by localised pain, spinal deformity (11) and may be associated with complications such as paraspinal abscesses, psoas abscesses (12) and neurological sequelae; and peripheral osteoarticular TB, which typically affects long weight-bearing bones causing localised bony pain, or it may cause joint pains in the context of TB osteomyelitis (13);
- TB peritonitis is characterised most commonly by abdominal pain, fever and weight loss. Other gastrointestinal manifestations include diarrhoea, ascites, hepatomegaly and splenomegaly (14);
- Genitourinary TB may result in dysuria, haematuria, flank pain, pelvic inflammatory disease and epidydimal masses depending on the specific site of infection (15);
- TB pericarditis is rare, characterised by symptoms of chest pain, cough and dyspnoea and clinical findings include fever, tachycardia, cardiomegaly and a pericardial rub (16).
Various factors increase the risk of contracting TB, as listed in Table 1 (17).
Microbiological investigations for pulmonary TB
International guidelines state that all patients suspected of having pulmonary TB infection should have at least two (ideally three) sputum samples sent for microscopic analysis and subsequent culture, with at least one sample sent in the early-morning as this is associated with the highest acid-fast bacilli (AFB) yield (18). If patients cannot produce sputum, the use of induced sputum techniques and bronchoscopy can be considered to obtain specimens (19). Smear-positive cases of TB, defined as cases where at least one sputum sample is positive for AFB, are the most infectious cases. They require particular attention to minimise infection transmission and appropriate contract tracing is required (20).
Radiological findings in pulmonary TB
Thoracic imaging is important both for diagnosing pulmonary TB and monitoring response to treatment (21).
The commonest radiographic findings for primary pulmonary TB include parenchymal disease, lymphadenopathy, pleural effusions and miliary disease (22). Homogenous parenchymal consolidation of the middle and lower lung lobes is suggestive of disease, although differential diagnoses would include a host of other bacterial infections (22). Unilateral right hilar and right paratracheal region lymphadenopathy are typical, although bilateral hilar lymphadenopathy is also common. CT evidence of intrathoracic lymph nodes with a diameter greater than 2 cm with central areas of low attenuation is suggestive of caseous necrosis and highly indicative of active TB disease (23). Pleural involvement has been reported in up to 38% of cases of primary pulmonary TB (24). High resolution CT scans of the chest are particularly sensitive at detecting miliary TB, which is characterised by diffuse 2–3 mm nodules throughout the lung parenchyma (25).
Post-primary TB, which refers to TB reinfection or reactivation and typically affects adults, is characterised by parenchymal disease which usually has a different radiographic distribution and appearance to that of primary TB. Parenchymal involvement in post-primary TB typically affects the lung apices and posterior segments of the upper lobes. Cavitation is the hallmark of post-primary TB (26). The presence of a ‘tree-in-bud’ appearance on CT, whereby multiple centrilobular nodules are connected to multiple linear branching opacities, may also be suggestive of TB, although it has been demonstrated in several other respiratory conditions (27). A combination of cavitation and ‘tree-in-bud’ is highly suggestive of TB. The majority of patients with endobronchial TB will have some degree of endobronchial stenosis (28) which will be demonstrable on CT imaging. Extension of TB disease to the pleura may result in multiseptated empyema, which has the potential to develop into a bronchopleural fistula (22).
Diagnosing drug-resistant TB
To establish the presence of drug resistance in M. tuberculosis isolates, WHO endorses the use of phenotypic drug susceptibility tests (DST) and nucleic acid amplification tests (NAATs) such as the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) and molecular line probe assays (LPAs) (29).
Phenotypic DST
Susceptibility testing for first- and second-line drugs can be undertaken using liquid cultures. While this approach is more sensitive than using solid cultures and offers a faster turnaround time for results, it can still take 2 weeks for pulmonary TB to be confirmed followed by a further 2 weeks to obtain the final DST result. Other drawbacks include the high equipment costs and the need to ensure that processing methods minimise the risk of culture contamination. Using solid cultures instead is cheaper, but confirmatory results take significantly longer: up to 8 weeks for pulmonary TB confirmation followed by 6 weeks to obtain the final DST results. Testing for DST to second-line drugs has also not been fully validated using the solid culture method (29). The significant time taken to yield final results by the conventional DST method carries a risk of patients with drug-resistant TB being treated with antibiotic regimens that may be ineffective, which may permit further spread of drug-resistant strains and potentially promote selection of strains with even greater resistance (30).
To address this problem, rapid diagnostic methods are needed. The microscopic-observation drug-susceptibility (MODS) assay is a faster, less expensive culture-based method that can be used instead of conventional culture methods to identify drug resistance (31). In this assay, liquid media either contain anti-TB drugs or are drug-free. After inoculation of the liquid media with sputum, light microscopy is used to detect the growth of M. tuberculosis colonies. The presence of colonies in only the drug-free liquid media indicates a positive culture result; the presence of colonies in both the drug-free and drug-containing liquid media indicates drug resistance (31,32). There is greater agreement between the MODS assay and conventional culture methods when testing for isolate susceptibility to rifampicin or isoniazid than when testing for susceptibility to ethambutol or streptomycin (33). A drawback of the MODS assay is the need to use an inverted microscope, which may not be routinely available in diagnostic laboratories (34). The use of the thin-layer agar (TLA) method circumvents this problem: it uses a solid medium, requires standard microscopy and has a higher sensitivity than conventional culture methods (35).
Xpert® MTB/RIF assays
The Xpert® MTB/RIF assay is an automated, real-time NAAT assay that allows both detection of M. tuberculosis and detection of mutations in the rpoB gene which confers M. tuberculosis with resistance to rifampicin (36). Results are available after approximately 90 minutes. The test has been shown to be sensitive, specific and cost-effective (37): it can detect 92.2% of culture positive patients and detects rifampicin resistance with a sensitivity of 99.1% (38). Nevertheless, its use in settings with low TB incidence has been questioned given the test’s lower sensitivity in the context of less extensive disease (39). In addition, there is a potential risk of a higher positive rate for drug resistance in low incidence settings (40). However, the test has recently been shown to have a very high negative predictive value in such settings (41).
In the UK, which is a low incidence setting, findings from one centre showed that discordance between the Xpert® MTB/RIF assay and culture results mainly occurred for non-respiratory specimens (42). However, considering that the culture confirmation rate is low in extrapulmonary TB, there is still a role for molecular tools like the Xpert® MTB/RIF assay (with the exception of pleural disease where its sensitivity is low compared to conventional culture) (43). The assay has been shown to be a useful adjunct in the diagnosis of mediastinal nodal TB using endobronchial ultrasound, particularly when used in conjunction with cytology obtained from transbronchial needle aspiration (44).
Molecular LPAs
Molecular LPAs are another type of genotypic test that allow rapid detection of M. tuberculosis and mutations that confer resistance to rifampicin and isoniazid (45). The mutations are detected in the rpoB, katG and inhA genes (46). A recent systematic review of three LPAs (Hain Genotype MTBDRplusV1, MTBDRplusV2 and Nipro NTM+MDRTB) has demonstrated that they detect rifampicin resistance with a pooled sensitivity of 96.7% [95% confidence interval (CI): 95.6–97.5%] and pooled specificity of 98.8% (95% CI: 98.2–99.2%), while they detect isoniazid resistance with a pooled sensitivity of 90.2% (95% CI: 88.2–91.9%) and pooled specificity of 99.2% (95% CI: 98.7–99.5%); furthermore the assays have a sensitivity of 94.4% in smear-positive samples but a sensitivity of 44% in smear-negative samples (47).
Identifying latent TB infection (LTBI)
Risk stratifying for LTBI
It has been estimated that one in three people globally is latently infected with TB (48) and that they have a 5–10% lifetime risk of progressing from LTBI to active TB disease (49). It would be neither practical nor clinically beneficial to identify and treat every individual with LTBI. Instead, guidelines call for a more pragmatic approach in which individuals considered to be at high-risk of having LTBI or at high-risk of converting from latent to active infection are targeted for testing and treatment. Those suspected of having LTBI should be asked about symptoms and chest radiography should be undertaken to exclude active disease.
WHO guidance on which groups to investigate and treat for LTBI in high-income and upper middle-income countries with a TB incidence less than 100 per 100,000 population is summarised in Table 2 (49). For resource-limited countries and those middle-income countries that do not fit into the previously described category, testing and treatment for LTBI is advised in all patients with HIV infection and children under 5 years of age who have been contacts of TB and who have been found not to have active TB (49). This is because these two groups have been shown to be at particularly high risk of developing active TB disease after being in contact with confirmed TB cases (50).

Full table
Diagnostic tests for LTBI
There is no agreed gold standard test for diagnosing LTBI. Indirect screening information is available from two types of test: tuberculin skins tests (TSTs) and interferon-gamma release assays (IGRAs).
Two widely used IGRAs are the QuantiFERON-TB Gold In-Tube (QFT-GIT) (Cellestis Ltd., Carnegie, Australia) and T-SPOT.TB (Oxford Immunotec Ltd., Oxford, UK). QFT-GIT is a whole-blood based enzyme-linked immunosorbent assay which measures the amount of interferon-gamma produced in response to exposure to the M. tuberculosis antigens ESAT-6, CFP-10 and TB7.7 (51). An upgraded version of this test has recently been introduced: the QuantiFERON-TB Plus (QFT-Plus) (Qiagen, Hilden, Germany) works in a similar way to QFT-GIT but differs because it contains an additional antigen tube that can stimulate both CD4 and CD8 T cells to produce interferon-gamma and it also dispenses with the TB7.7 antigen (52). T-SPOT.TB is an enzyme-linked immunospot-based test which measures the number of peripheral mononuclear cells producing interferon-gamma in response to ESAT-6 and CFP-10 (51). In resource-limited countries, WHO advises that IGRA should not replace the use of TST; this decision takes account of the performance and cost of the test in these settings (49,51). In high-income and upper middle-income countries, either TST or IGRA can be used (49).
TSTs may give false positive results if there is a history of BCG vaccination or exposure to non-tuberculous mycobacteria (53). False negative results may occur due to: patients being immunocompromised secondary to HIV infection, malignancy or use of immunosuppressant agents; extremes of patient age; improper administration of the tuberculin antigen (54). In patients with HIV infection, the decreased sensitivity of TST testing has been attributed to the phenomenon of anergy, which refers to a diminished delayed-type hypersensitivity response in previously sensitised individuals (55). Furthermore, repeated TST testing is associated with problems such as: boosting, where the immunological recall to previous exposure to mycobacterial antigens causes the TST response to increase; conversion of negative results to positive results due to new infections; reversion of positive results to negative results; and issues relating to variable biological response and variability among clinicians in reading the final results (56).
IGRAs are not affected by previous BCG vaccination or most non-tuberculous infections, which reduces the likelihood of false positive results compared to TSTs (57), although cross-reactivity has been demonstrated between IGRAs and both Mycobacterium marinum and Mycobacterium kansasii (58). IGRAs show variability in the reproducibility of their results even when the tests are conducted under identical conditions (59) and a significant number of conversions and reversions occur upon repeat testing (60). Potential sources of variability in IGRAs include the manufacturing source, pre-analytical sources (such as delay in incubation), analytical sources (caused by inter-operator and inter-laboratory imprecision) and immunological sources (such as boosting caused by purified protein derivative) (61).
Agreement between QFT-GIT and TST has been shown to increase as TST diameter increases (62). However, neither TSTs nor IGRAs can distinguish between latent and active TB infection (63). There is no significant difference between TST and QFT-GIT at detecting LTBI in children; nor is there any significant difference TST and T-SPOT.TB at detecting LTBI in immunocompromised patients (64). Although neither TSTs nor IGRAs have a sufficiently high accuracy to predict progression from LTBI to active disease (65), individuals with a TST induration
Recently there has been interest in understanding how the immunological signature of LTBI may be a predictor for progression to active TB as this may have implications for targeted LTBI treatment. A study has shown that recently acquired LTBI has a cellular immune signature that is more similar to the signature found in patients with active TB than in those with distantly acquired LTBI (67).
Clinical management
Treatment of drug-sensitive TB
First-line treatment of drug-sensitive TB is divided into a two-month intensive phase of treatment comprised of rifampicin, isoniazid, pyrazinamide and ethambutol followed by a four-month continuation phase consisting of rifampicin and isoniazid. Table 3 shows the recommended daily dosing of each of these drugs (20). Four-drug fixed dose combination regimens have been shown to have a similar efficacy to separately administered drugs (68) and the use of the former has been advocated as the prospect of taking fewer tablets may improve medication adherence among patients (20). In extra-pulmonary TB, the duration of therapy varies depending on the site of TB infection (69). For example, treatment for TB meningitis may be for up to 12 months (70).
There are various practical considerations in the administration of anti-TB drugs. As with all drugs, the safety profile, side effects and potential for interactions with other medications needs to be considered. Baseline and monitoring tests include blood tests to monitor liver function and assessment of visual acuity (71). Drug-induced liver injury from anti-TB medications is a particular concern. A recent retrospective study from one centre in the UK showed that almost 7% of patients started on anti-TB treatment developed drug-induced liver injury, but only a quarter of these met national criteria for liver function monitoring (72). A policy of checking liver function 2 weeks after starting anti-TB treatment has been advocated rather than purely using a risk-factor based approach to monitoring liver function (73).
The first-line anti-TB drugs may cause a range of adverse effects. Rifampicin is associated with hepatitis, gastrointestinal symptoms, pruritus, thrombocytopaenia, haemolytic anaemia and red discolouration of body secretions. It is a hepatic enzyme inducer that increases the metabolism of various drugs and is associated with a risk of postnatal haemorrhage in pregnant women. Isoniazid is associated with hepatitis, peripheral neuropathy (which may be prevented by supplementation with pyridoxine) and lupus-like syndrome. It is a hepatic enzyme inhibitor which increases the toxicity of multiple drugs. Pyrazinamide may be complicated by hepatitis, gastrointestinal disturbances, gout, arthralgia, sideroblastic anaemia and photosensitive dermatitis. Ethambutol may cause optic neuritis and arthralgia. The potential for complications demands close monitoring of patients during anti-TB therapy (20).
European guidelines recommend that a test of cure is undertaken during the continuation phase of treatment to demonstrate conversion to a culture-negative state (74). However, the appropriateness of this has been questioned in low incidence settings where patients are clinically well at the end of treatment, as further testing may be unnecessarily invasive (75).
Treatment of drug-resistant TB
An assessment should be made of the potential for drug resistance. This can be both mono-resistance, such as isoniazid-resistance or rifampicin-resistance, or MDR TB. Any assessment should utilise information on the background prevalence of drug-resistant TB, possible exposure to drug-resistant TB and its development through poor medication adherence.
Revised WHO guidelines for MDR TB
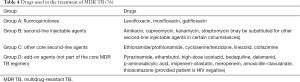
In 2016, WHO updated its guidance on the treatment of MDR TB. It was acknowledged that the quality of evidence for the effectiveness of many medications is low due to most studies being observational in design and there being a paucity of randomised controlled trials. The groups of drugs used to treat MDR TB are summarised in Table 4 (76).
The guidelines for the revised MDR TB regimens have been based on two sources: a meta-analysis of three systematic reviews; and evidence from unpublished data in a meta-analysis undertaken by WHO (77,78). MDR TB regimens should now consist of at least five agents: pyrazinamide (unless there is confirmed pyrazinamide-resistance) and four core-second line drugs (groups A–C in Table 4). Such regimens seek to improve cure rates and mortality rates while decreasing spread of further resistance (76). The standard duration for conventional MDR TB treatment is 20–24 months. A retrospective study of MDR TB cases in the UK has shown that MDR TB treatment regimens are associated with successful outcomes but initial hospital admissions are prolonged and patient adherence to treatment is a limiting factor (79).
Fluoroquinolones in particular are now regarded as the most important component of the MDR TB regimen (76). Their utility in treating MDR TB and in shortening the length of treatment for drug-sensitive TB has been the focus of much research (80). Bedaquiline (TMC207), a diarylquinoline which inhibits adenosine triphosphate (ATP) synthase in M. tuberculosis (81), was granted accelerated approval for use in the treatment of MDR TB by the US Food and Drug Administration in 2012 (82) and WHO issued interim guidance on its use in 2013 (83). A review of bedaquiline was commissioned by WHO in 2016 to evaluate the latest evidence for its use in MDR TB; no changes to the 2013 guidelines were recommended (84). Delamanid (OPC-67683), a nitro-dihydro-imidazooxazole derivative which inhibits mycolic acid synthesis (85), was approved by WHO in 2014 for use in adults with MDR TB (86). In 2016, WHO issued recommendations for its use in paediatric patients with MDR TB (87). In both population groups, patients should meet strict inclusion criteria and treatment requires close monitoring (86,87).
The list of side effects for second-line agents is extensive. For example, ototoxicity is a significant problem with second-line injectable agents (88). Various resources may be consulted for information on the important adverse effects, interactions and monitoring requirements of drugs used in treating MDR TB (71,89). WHO guidelines provide important information on the need for adequate nutritional support, careful monitoring of patients taking anti-retroviral therapy for HIV coinfection and the role of surgical intervention in patients with MDR TB (90).
Shortened MDR TB regimen
Shortening the duration of treatment while retaining efficacy is naturally desirable. In 2016, WHO issued conditional recommendations on a shortened treatment regimen for MDR TB, which comprises initial treatment for 4–6 months with kanamycin, moxifloxacin, prothionamide, clofazimine, pyrazinamide, high-dose isoniazid and ethambutol followed by 5 months of moxifloxacin, clofazimine, pyrazinamide and ethambutol. Exclusion criteria for the shortened regimen are as follows: confirmed resistance to or suspected ineffectiveness of drugs in the shortened regimen (excluding isoniazid resistance); exposure to one or more second-line drugs in the shortened regimen for more than one month; intolerance to one or more drugs in the shortened regimen; risk of toxicity from the drugs used; pregnancy; extrapulmonary disease; and lack of availability of one of the drugs in the regimen (91).
The shortened regimen was derived from a study that trialled the so-called ‘Bangladesh regimen’, in which 206 MDR TB patients were treated with gatifloxacin, clofazimine, ethambutol and pyrazinamide for 9 months alongside prothionamide, kanamycin and high-dose isoniazid for a minimum of the first 4 months of treatment; a cure rate of 87.9% (95% CI: 82.7–91.6%) was reported (92). A follow-up to this study involving 515 patients subsequently demonstrated a favourable outcome in 84.4% of cases treated with the shortened MDR TB treatment regimen and showed that fluoroquinolone resistance was the most important risk factor for unfavourable outcomes, particularly when compounded by additional resistance to pyrazinamide (93).
Although the shortened MDR TB regimen has been advocated for use by the British Thoracic Society (BTS), careful selection and monitoring of outcomes of appropriate patients for the shorter regimen is recommended given the differing patterns of drug resistance within the UK. The BTS guidance also takes account of local supply issues: for example, kanamycin is not easily available in the UK and the use of amikacin or capreomycin in its place is advised (94). A range of logistical challenges relating to implementation of the shortened regimen remain to be addressed in low-income countries (95). An important issue is whether countries have the resources to test for resistance against all the drugs in the regimen (96). Furthermore, a large proportion of MDR TB patients may not be eligible for the shortened regimen, as it has been demonstrated that more than 40% of cases may demonstrate resistance to fluoroquinolones or kanamycin (97). This raises the question as to what drugs can be safely and effectively substituted into the regimen should one of the core drugs be ineffective (96).
The first randomised controlled trial evaluating the shortened MDR TB treatment regimen is currently underway. Stage 1 of the STREAM (Evaluation of a Standard Treatment Regimen of Anti-tuberculosis Drugs for Patients with Multidrug-resistant Tuberculosis) is evaluating the efficacy and safety of the regimen while stage 2 examines regimens that include bedaquiline (98). Other novel short treatment regimens are also under investigation and have shown promising results thus far: the combination of pretomanid (PA-824), moxifloxacin and pyrazinamide has shown favourable outcomes when used in treatment of drug-susceptible and MDR TB for 8 weeks in a phase 2b trial (99); a phase 3 trial entitled STAND (Shortening Treatment by Advancing Novel Drugs) is now underway (100). The results of these trials will provide important evidence regarding the clinical utility of shortened MDR TB treatment regimens.
Treatment of LTBI
Successful screening for LTBI plays a crucial role in identifying and treating individuals who might otherwise go on to develop active disease (101). Screening and treating new migrants to the UK from countries with high TB prevalence has been shown to be cost-effective (102).
WHO guidelines recommend any of the following treatment options for LTBI (49):
- 6 months of isoniazid monotherapy;
- 9 months of isoniazid monotherapy;
- 3 months of weekly rifapentine plus isoniazid;
- 3–4 months of isoniazid plus rifampicin;
- 3–4 months of rifampicin monotherapy.
The recommendations were made based on a meta-analysis evaluating the efficacy and safety of various treatment regimens for LTBI (103). While WHO panel members agreed unanimously that the first three options were equivalent, there was less agreement regarding the equivalence of the final two regimens (49). Additionally, the 6-month isoniazid regimen was associated with more hepatotoxic events than the 3- to 4-month rifampicin regimen; and the 9-month isoniazid regimen was associated with more hepatotoxic events than the 3-month weekly rifapentine plus isoniazid regimen (49).
Future priorities
There have clearly been significant advances in approaches to the diagnosis and treatment of TB. However, there is still much progress to be made.
Recognising those patients who are at greatest risk of converting from latent to active TB will enable a more targeted approach to LTBI treatment. Treating before conversion to active disease will not only reduce patient morbidity but also have important long-term cost saving implications. To achieve this, further research and improvements in the efficacy and interpretation of diagnostic tools are needed. This is already underway. In the UK, the PREDICT trial, which is a study of the efficacy of IGRAs compared to TSTs at predicting conversion to active TB disease in 10,000 participants, is due to report imminently (104). Importantly, even if TST or IGRA testing is negative among contacts of confirmed TB cases, such contacts should still be advised to rapidly engage with healthcare services in the future should symptoms develop (105).
Furthermore, studies have started evaluating the clinical utility of the recently developed QFT-Plus, which has been shown to have a stronger association with surrogate markers of exposure to TB than QFT-GIT in the context of contact screening, although the absence of a gold-standard for diagnosing LTBI makes the discordance between QFT-GIT and QFT-Plus difficult to evaluate (106). Nevertheless, the findings suggest a possible role for QFT-Plus in identifying patients at increased risk of conversion to active disease (105). In a study among healthcare workers in a low TB incidence setting, QFT-Plus and QFT-GIT were shown to have a high degree of agreement (107). In another study in patients with active TB, QFT-Plus sensitivity has been shown to be lower among HIV patients with severe immunosuppression (108). Further large-scale studies and comparators to the T-SPOT.TB test are needed.
Recently a new approach to diagnosing TB infection using a novel skin test has been evaluated. C-Tb is a M. tuberculosis-specific skin test containing the antigens ESAT-6 and CFP-10. Although it has been shown to have a similar sensitivity to QFT-GIT, there was discordance between the two tests, which was attributed to the different antigen makeup of the tests and the different immune responses that they subsequently elicit; additionally, the sensitivity of C-Tb was reduced in HIV-infected patients who were severely immunocompromised (109). Its utility in clinical practice remains to be determined.
There have also been developments in diagnostic tests for MDR TB. The Xpert® MTB/RIF Ultra assay has recently been endorsed by WHO as a non-inferior option to using the Xpert® MTB/RIF assay. To improve sensitivity for detecting M. tuberculosis, the Xpert® MTB/RIF Ultra assay incorporates two different amplification targets and a larger DNA reaction chamber. To improve detection of rifampicin resistance, the assay uses melting temperature-based analysis instead of real-time polymerase chain reaction (PCR) (110). A multicentre study found that the Xpert® MTB/RIF Ultra assay has a higher sensitivity than the Xpert® MTB/RIF assay, particularly among smear-negative patients and those with HIV coinfection, and that rifampicin resistance detection was at least as good as that achieved by the Xpert® MTB/RIF assay. However, the higher sensitivity of Xpert® MTB/RIF Ultra comes at the expense of its specificity, which is lower than that of Xpert® MTB/RIF (111).
Research is already underway into the use of next-generation sequencing (NGS) to expedite the diagnosis of TB. Whole-genome sequencing (WGS) can be used to identify mutations conferring drug susceptibility as well as drug resistance (112). Furthermore, NGS can be used to identify the extent to which specific mutations affect an isolate’s resistance to particular drugs; that is, the effect of a specific mutation on the minimum inhibitory concentration (MIC) can be determined (113). For example, a mutation in the katG gene at S315T is associated with high level isoniazid resistance (MIC 2–8 micrograms/mL); while a mutation in the inhA promoter results in low level isoniazid resistance (MIC 0.2–0.5 micrograms/mL) (114,115). NGS has also shown that key genes in TB demonstrate heteroresistance, a phenomenon whereby incomplete gene mutations result in isolate cultures that simultaneously grow drug-susceptible and drug-resistant colonies: NGS is particularly sensitive at detecting pncA heteroresistance which determines the degree of resistance to pyrazinamide (116). The clinical utility of WGS in determining anti-TB drug resistance profiles directly from sputum samples has already been demonstrated (117). However, standardisation and validation of DNA extraction from sputum samples will be required if widespread uptake of WGS in TB diagnostics is to be achieved (118).
Many challenges also remain in improving treatment protocols and outcomes. The complexity of treating MDR TB has already been addressed, but a further issue is the growing threat of extensively drug-resistant (XDR) TB, which is caused by M. tuberculosis isolates resistant to rifampicin, isoniazid, fluoroquinolones and at least one of the second-like injectable drugs. One hundred and seventeen countries had reported cases of XDR TB by the end of 2015: 9.5% of MDR TB cases had XDR TB (1). Various trials evaluating effective treatment protocols for XDR TB are currently underway: the NiX-TB trial is evaluating the use of a 6-month regimen of pretomanid (PA-824), bedaquiline and linezolid; the TB-PRACTECAL trial is evaluating the use of regimens containing bedaquiline, pretomanid (PA-824) and linezolid, with or without clofazimine or moxifloxacin (119).
The utility of other novel therapies to treat TB is also the focus of current research. The role of vitamin D deficiency in the pathogenesis of TB has been under investigation for some time: low vitamin D has been associated with both active TB and LTBI and it has been implicated as a risk factor for extrapulmonary TB (120,121). However, evidence for the role of vitamin D replacement in TB treatment is conflicting. While it has been argued that vitamin D accelerates recovery from tuberculosis (122), this notion has been challenged in various studies (123,124). The value of vitamin D replacement in TB requires further research.
Curtailing the TB crisis globally requires countries to have a strategy to provide coordinated and integrated care at a national level (125). Diagnostic tests need to be both clinically effective and cost-effective if they are to be implemented in national programmes. Treatment regimens need to be safe, efficacious and tolerable to patients. Developing shorter, more effective regimens with fewer adverse effects will promote adherence to therapy. Additionally, eliminating TB will require consideration of other factors that have not been discussed in this review, such as improved efficacy of anti-TB vaccinations; robust strategies for HIV testing, treatment and monitoring; economic support for lower-income countries in establishing high-quality, sustainable facilities for TB diagnosis and treatment; and tailoring services for those with certain social risk factors such as homelessness and drug abuse (126,127). Furthermore, given that TB incidence is greatest among those originating from high TB burden settings (128-130), implementation of effective screening programmes for new migrants is important. For these goals to be achieved, greater investment in health and social care infrastructure as well as engagement with the private sector will be necessary (131). Ultimately, eradicating TB in the coming decades will require a globally coordinated programmatic approach to address the current deficits in strategies for TB prevention, diagnosis and treatment (132).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organisation. WHO Global Tuberculosis Report 2016. WHO, 2016. Accessed on 1st July 2017. Available online: http://www.who.int/tb/publications/global_report/en/
- World Health Organisation. The End TB Strategy. Global strategy and targets for tuberculosis prevention, care and control after 2015. WHO, 2016. Accessed on 1st July 2017. Available online: http://www.who.int/tb/post2015_TBstrategy.pdf?ua=1
- Mack U, Migliori GB, Sester M, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009;33:956-73. [Crossref] [PubMed]
- Lawn SD, Zumla AI. Tuberculosis. Lancet 2011;378:57-72. [Crossref] [PubMed]
- Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician 2005;72:1761-8. [PubMed]
- Artenstein AW, Kim JH, Williams WJ, et al. Isolated peripheral tuberculous lymphadenitis in adults: current clinical and diagnostic issues. Clin Infect Dis 1995;20:876-82. [Crossref] [PubMed]
- Polesky A, Grove W, Bhatia G. Peripheral tuberculous lymphadenitis: epidemiology, diagnosis, treatment, and outcome. Medicine (Baltimore) 2005;84:350-62. [Crossref] [PubMed]
- Berger HW, Mejia E. Tuberculous Pleurisy. Chest 1973;63:88-92. [Crossref] [PubMed]
- Rock RB, Olin M, Baker CA, et al. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev 2008;21:243-61. [Crossref] [PubMed]
- Sütlaş PN, Unal A, Forta H, et al. Tuberculous meningitis in adults: review of 61 cases. Infection 2003;31:387-91. [PubMed]
- Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med 2011;34:440-54. [Crossref] [PubMed]
- Harrigan RA, Kauffman FH, Love MB. Tuberculous psoas abscess. J Emerg Med 1995;13:493-8. [Crossref] [PubMed]
- Pigrau-Serrallach C, Rodríguez-Pardo D. Bone and joint tuberculosis. Eur Spine J 2013;22 Suppl 4:556-66. [Crossref] [PubMed]
- Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther 2005;22:685-700. [Crossref] [PubMed]
- Kapoor R, Ansari MS, Mandhani A, et al. Clinical presentation and diagnostic approach in cases of genitourinary tuberculosis. Indian J Urol 2008;24:401-5. [Crossref] [PubMed]
- Trautner BW, Darouiche RO. Tuberculous pericarditis: optimal diagnosis and management. Clin Infect Dis 2001;33:954-61. [Crossref] [PubMed]
- Narasimhan P, Wood J, Macintyre CR, et al. Risk factors for tuberculosis. Pulm Med 2013;2013:828939.
- Tuberculosis Coalition for Technical Assistance. International Standards for Tuberculosis Care (ISTC), 2nd edition. Tuberculosis Coalition for Technical Assistance, The Hague, 2009.
- Conde MB, Soares DL, Mello FC, et al. Comparison of sputum induction with fiberoptic bronchoscopy in the diagnosis of tuberculosis: experience at an acquired immune deficiency syndrome reference center in Rio de Janeiro, Brazil. Am J Respir Crit Care Med 2000;162:2238-40. [Crossref] [PubMed]
- World Health Organisation. Treatment of tuberculosis: guidelines – 4th edition. WHO, 2010. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&ua=1
- Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis 2015;32:87-93. [Crossref] [PubMed]
- Burrill J, Williams CJ, Bain G, et al. Tuberculosis: a radiologic review. Radiographics 2007;27:1255-73. [Crossref] [PubMed]
- Curvo-Semedo L, Teixeira L, Caseiro-Alves F. Tuberculosis of the chest. Eur J Radiol 2005;55:158-72. [Crossref] [PubMed]
- Woodring JH, Vandiviere HM, Fried AM, et al. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986;146:497-506. [Crossref] [PubMed]
- Leung AN. Pulmonary tuberculosis: the essentials. Radiology 1999;210:307-22. [Crossref] [PubMed]
- Andreu J, Cáceres J, Pallisa E, et al. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol 2004;51:139-49. [Crossref] [PubMed]
- Rossi SE, Franquet T, Volpacchio M, et al. Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2005;25:789-801. [Crossref] [PubMed]
- Han JK, Im JG, Park JH, et al. Bronchial stenosis due to endobronchial tuberculosis: successful treatment with self-expanding metallic stent. AJR Am J Roentgenol 1992;159:971-2. [Crossref] [PubMed]
- World Health Organisation. Guidelines for surveillance of drug resistance in tuberculosis – 5th edition. WHO, 2015. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/174897/1/9789241549134_eng.pdf?ua=1
- Kumar K, Abubakar I. Clinical implications of the global multidrug-resistant tuberculosis epidemic. Clin Med (Lond) 2015;15 Suppl 6:s37-42. [Crossref] [PubMed]
- Minion J, Leung E, Menzies D, et al. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:688-98. [Crossref] [PubMed]
- Caviedes L, Lee TS, Gilman RH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. The Tuberculosis Working Group in Peru. J Clin Microbiol 2000;38:1203-8. [PubMed]
- Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 2006;355:1539-50. [Crossref] [PubMed]
- Palomino JC, Martin A, Portaels F. MODS assay for the diagnosis of TB. N Engl J Med 2007;356:188; author reply 189. [Crossref] [PubMed]
- Robledo JA, Mejía GI, Morcillo N, et al. Evaluation of a rapid culture method for tuberculosis diagnosis: a Latin American multi-center study. Int J Tuberc Lung Dis 2006;10:613-9. [PubMed]
- Weyer K, Mirzayev F, Migliori GB, et al. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J 2013;42:252-71. [Crossref] [PubMed]
- Drobniewski F, Cooke M, Jordan J, et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess 2015;19:1-188. vii-viii. [Crossref] [PubMed]
- World Health Organisation. Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO, 2011. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/44586/1/9789241501545_eng.pdf?ua=1&ua=1
- Sohn H, Aero AD, Menzies D, et al. Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin Infect Dis 2014;58:970-6. [Crossref] [PubMed]
- Mokaddas E, Ahmad S, Eldeen HS, et al. Discordance between Xpert MTB/RIF assay and Bactec MGIT 960 Culture System for detection of rifampin-resistant Mycobacterium tuberculosis isolates in a country with a low tuberculosis (TB) incidence. J Clin Microbiol 2015;53:1351-4. [Crossref] [PubMed]
- Parcell BJ, Jarchow-MacDonald AA, Seagar AL, et al. Three year evaluation of Xpert MTB/RIF in a low prevalence tuberculosis setting: A Scottish perspective. J Infect 2017;74:466-72. [Crossref] [PubMed]
- Singanayagam A, Donaldson H, Kon OM. GeneXpert(®) MTB/RIF in low prevalence settings: a UK laboratory perspective. J Infect 2014;69:199-200. [Crossref] [PubMed]
- Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014;44:435-46. [Crossref] [PubMed]
- Dhasmana DJ, Ross C, Bradley CJ, et al. Performance of Xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Ann Am Thorac Soc 2014;11:392-6. [Crossref] [PubMed]
- World Health Organisation. Policy statement: molecular line probe assays for rapid screening of patients at risk of multi-drug resistant tuberculosis (MDR-TB). WHO, 2008. Accessed on 1st July 2017. Available online: http://www.who.int/tb/features_archive/policy_statement.pdf?ua=1
- Telenti A, Honore N, Bernasconi C, et al. Genotypic assessment of isoniazid and rifampin resistance in Mycobacterium tuberculosis: a blind study at reference laboratory level. J Clin Microbiol 1997;35:719-23. [PubMed]
- Nathavitharana RR, Cudahy PG, Schumacher SG, et al. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J 2017;49. [Crossref] [PubMed]
- Dye C, Scheele S, Dolin P, et al. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999;282:677-86. [Crossref] [PubMed]
- World Health Organisation. Guidelines on the management of latent tuberculosis infection. WHO, 2015. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/136471/1/9789241548908_eng.pdf?ua=1&ua=1
- Fox GJ, Barry SE, Britton WJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013;41:140-56. [Crossref] [PubMed]
- World Health Organisation. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries: policy statement. WHO, 2011. Accessed on 1st July 2017. Available online: http://www.who.int/tb/features_archive/policy_statement_igra_oct2011.pdf
- QIAGEN. QuantiFERON-TB Gold Plus (QFT-Plus). Accessed on 1st July 2017. Available online: https://www.qiagen.com/gb/shop/detection-solutions/human-pathogens/quantiferon-tb-gold-plus/#productdetails
- Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and nontuberculous mycobacteria? Int J Tuberc Lung Dis 2006;10:1192-204. [PubMed]
- Dunlap NE, Bass J, Fujiwara P, et al. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am J Respir Crit Care Med 2000;161:1376-95. [Crossref] [PubMed]
- Cobelens FG, Egwaga SM, van Ginkel T, et al. Tuberculin skin testing in patients with HIV infection: limited benefit of reduced cutoff values. Clin Infect Dis 2006;43:634-9. [Crossref] [PubMed]
- Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med 1999;159:15-21. [Crossref] [PubMed]
- Stout JE. Predicting tuberculosis: does the IGRA tell this tale? Am J Respir Crit Care Med 2008;177:1055-7. [Crossref] [PubMed]
- Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis 2002;186:1797-807. [Crossref] [PubMed]
- Tagmouti S, Slater M, Benedetti A, et al. Reproducibility of interferon gamma (IFN-γ) release assays. A systematic review. Ann Am Thorac Soc 2014;11:1267-76. [Crossref] [PubMed]
- Pai M, O'Brien R. Serial testing for tuberculosis: can we make sense of T cell assay conversions and reversions? PLoS Med 2007;4:e208. [Crossref] [PubMed]
- Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014;27:3-20. [Crossref] [PubMed]
- Kruczak K, Mastalerz L, Sladek K. Interferon-gamma release assays and tuberculin skin testing for diagnosing latent Mycobacterium tuberculosis infection in at-risk groups in Poland. Int J Mycobacteriol 2016;5:27-33. [Crossref] [PubMed]
- Sester M, Sotgiu G, Lange C, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 2011;37:100-111. [Crossref] [PubMed]
- Auguste P, Tsertsvadze A, Pink J, et al. Comparing interferon-gamma release assays with tuberculin skin test for identifying latent tuberculosis infection that progresses to active tuberculosis: systematic review and meta-analysis. BMC Infect Dis 2017;17:200. [Crossref] [PubMed]
- Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:45-55. [Crossref] [PubMed]
- Altet N, Dominguez J, Souza-Galvão ML, et al. Predicting the development of tuberculosis with the tuberculin skin test and QuantiFERON testing. Ann Am Thorac Soc 2015;12:680-8. [Crossref] [PubMed]
- Halliday A, Whitworth H, Kottoor SH, et al. Stratification of latent mycobacterium tuberculosis infection by cellular immune profiling. J Infect Dis 2017;215:1480-7. [Crossref] [PubMed]
- Lienhardt C, Cook SV, Burgos M, et al. Efficacy and safety of a 4-drug fixed-dose combination regimen compared with separate drugs for treatment of pulmonary tuberculosis: the Study C randomized controlled trial. JAMA 2011;305:1415-23. [Crossref] [PubMed]
- Pusch T, Pasipanodya JG, Hall RG 2nd, et al. Therapy duration and long-term outcomes in extra-pulmonary tuberculosis. BMC Infect Dis 2014;14:115. [Crossref] [PubMed]
- Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society / Centers for Disease Control and Prevention / Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis 2016;63:853-67. [Crossref] [PubMed]
- Potter JL, Capstick T, Ricketts WM, et al. A UK-based resource to support the monitoring and safe use of anti-TB drugs and second-line treatment of multidrug-resistant TB. Thorax 2015;70:297-8. [Crossref] [PubMed]
- Abbara A, Chitty S, Roe JK, et al. Drug-induced liver injury from antituberculosis treatment: a retrospective study from a large TB centre in the UK. BMC Infect Dis 2017;17:231. [Crossref] [PubMed]
- Singanayagam A, Sridhar S, Dhariwal J, et al. A comparison between two strategies for monitoring hepatic function during antituberculous therapy. Am J Respir Crit Care Med 2012;185:653-9. [Crossref] [PubMed]
- Migliori GB, Zellweger JP, Abubakar I, et al. European union standards for tuberculosis care. Eur Respir J 2012;39:807-19. [Crossref] [PubMed]
- Leahy AU, Lipman M, Hetzel M, et al. Do we need bacteriological confirmation of cure in uncomplicated tuberculosis? Eur Respir J 2013;42:860-3. [Crossref] [PubMed]
- World Health Organisation. WHO treatment guidelines for drug-resistant tuberculosis – 2016 update. WHO, 2016. Accessed on 1st July 2017. Available online: http://www.who.int/tb/areas-of-work/drug-resistant-tb/MDRTBguidelines2016.pdf
- Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012;9:e1001300. [Crossref] [PubMed]
- World Health Organisation. WHO treatment guidelines for drug-resistant tuberculosis. 2016 update. Annexes 4, 5 and 6. WHO, 2016. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/250125/5/9789241549639-webannexes-eng.pdf?ua=1
- Arnold A, Cooke GS, Kon OM, et al. Drug resistant TB: UK multicentre study (DRUMS): Treatment, management and outcomes in London and West Midlands 2008-2014. J Infect 2017;74:260-71. [Crossref] [PubMed]
- Kumar K, McHugh TD, Lipman M. Fluoroquinolones for treating tuberculosis. Clinical Pharmacist 2017;9:142-9.
- Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005;307:223-7. [Crossref] [PubMed]
- Mahajan R. Bedaquiline: First FDA-approved tuberculosis drug in 40 years. Int J Appl Basic Med Res 2013;3:1-2. [Crossref] [PubMed]
- World Health Organisation. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis. Interim policy guidance. Geneva: WHO, 2013. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/84879/1/9789241505482_eng.pdf
- Mbuagbaw L. Review of available evidence on the use of bedaquiline for the treatment of multidrug-resistant tuberculosis: Data analysis report. WHO, 2016. Accessed on 1st July 2017. Available online: http://www.who.int/tb/publications/2017/Appendix_GDGReport_Bedaquiline.pdf?ua=1
- Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 2006;3:e466. [Crossref] [PubMed]
- World Health Organisation. The use of delamanid in the treatment of multidrug-resistant tuberculosis. Interim policy guidance. WHO, 2014. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/137334/1/WHO_HTM_TB_2014.23_eng.pdf
- World Health Organisation. The use of delamanid in the treatment of multidrug-resistant tuberculosis in children and adolescents. Interim policy guidance. WHO, 2016. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/250614/1/9789241549899-eng.pdf
- Arnold A, Cooke GS, Kon OM, et al. The adverse effects and choice of injectable agents in MDR-TB: amikacin or capreomycin. Antimicrob Agents Chemother. 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Potter JL, Capstick T, Ricketts WMR, et al. A UK based resource to support the monitoring and safe use of anti-tuberculosis drugs and second line treatment of multidrug-resistant tuberculosis. TB Drug Monographs, 2015. Accessed on 1st July 2017. Available online: http://www.tbdrugmonographs.co.uk/
- World Health Organisation. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO, 2014. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf?ua=1&ua=1
- World Health Organisation. The shorter MDR-TB regimen. WHO, 2016. Accessed on 1st July 2017. Available online: http://www.who.int/tb/Short_MDR_regimen_factsheet.pdf
- Van Deun A, Maug AK, Salim MA, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2010;182:684-92. [Crossref] [PubMed]
- Aung KJM, Van Deun A, Declercq E, et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 2014;18:1180-7. [Crossref] [PubMed]
- Bothamley G, Dedicoat M, Kon OM, et al. The Management of Multidrug-resistant tuberculosis (MDRTB). British Thoracic Society, 2016. Accessed on 1st July 2017. Available online: http://www.brit-thoracic.org.uk/document-library/guidelines-and-quality-standards/clinical-statements/bts-clinical-statement-management-of-mdrtb/
- Sloan DJ, Lewis JM. Management of multidrug-resistant TB: novel treatments and their expansion to low resource settings. Trans R Soc Trop Med Hyg 2016;110:163-72. [Crossref] [PubMed]
- Sotgiu G, Tiberi S, Centis R, et al. Applicability of the shorter ‘Bangladesh regimen’ in high multidrug-resistant tuberculosis settings. Int J Infect Dis 2017;56:190-3. [Crossref] [PubMed]
- Sotgiu G, Tiberi S, D’Ambrosio L, et al. Faster for less: the new “shorter” regimen for multidrug-resistant tuberculosis. Eur Respir J 2016;48:1503-7. [Crossref] [PubMed]
- Moodley R, Godec TR. STREAM Trial Team. Short-course treatment for multidrug-resistant tuberculosis: the STREAM trials. Eur Respir Rev 2016;25:29-35. [Crossref] [PubMed]
- Dawson R, Diacon AH, Everitt D, et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet 2015;385:1738-47. [Crossref] [PubMed]
- Global Alliance for TB Drug Development. Shortening Treatment by Advancing Novel Drugs (STAND). ClinicalTrials.gov – A service of the U.S. National Institutes of Health. Accessed on 1st July 2017. Available online: http://clinicaltrials.gov/ct2/show/NCT02342886
- Kon OM. Time for a preventative strategy for TB in the UK: further evidence for new entrant screening in primary care. Thorax 2014;69:305-6. [Crossref] [PubMed]
- Pareek M, Watson JP, Ormerod LP, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 2011;11:435-44. [Crossref] [PubMed]
- Stagg HR, Zenner D, Harris RJ, et al. Treatment of latent tuberculosis infection – a network meta-analysis. Ann Intern Med 2014;161:419-28. [Crossref] [PubMed]
- National Institute for Health Research. HTA – 08/68/01. Prognostic value of interferon gamma release assays in predicting active tuberculosis among individuals with, or at risk of, latent tuberculosis infection. NIHR. Accessed on 1st July 2017. Available online: http://www.journalslibrary.nihr.ac.uk/programmes/hta/086801#/
- Kumar K, Shorten RJ, Capocci S, et al. The value of “inform and advise” guidance in a case of extensive tuberculosis transmission. J Infect 2013;67:158-60. [Crossref] [PubMed]
- Barcellini L, Borroni E, Brown J, et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 2016;48:1411-9. [Crossref] [PubMed]
- Moon HW, Gaur RL, Tien SS, et al. Evaluation of QuantiFERON-TB Gold-Plus in Health Care Workers in a Low-Incidence Setting. J Clin Microbiol 2017;55:1650-7. [Crossref] [PubMed]
- Telisinghe L, Amofa-Sekyi M, Maluzi K, et al. The sensitivity of the QuantiFERON®-TB Gold Plus assay in Zambian adults with active tuberculosis. Int J Tuberc Lung Dis 2017;21:690-6. [Crossref] [PubMed]
- Hoff ST, Peter JG, Theron G, et al. Sensitivity of C-Tb: a novel RD-1-specific skin test for the diagnosis of tuberculosis infection. Eur Respir J 2016;47:919-28. [Crossref] [PubMed]
- World Health Organisation. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. WHO, 2017. Accessed on 1st July 2017. Available online: http://apps.who.int/iris/bitstream/10665/254792/1/WHO-HTM-TB-2017.04-eng.pdf?ua=1
- FIND Study Team. Report for WHO. A multicentre non-inferiority diagnostic accuracy study of the Ultra assay compared to the Xpert MTB/RIF assay. FIND, 2017. Accessed on 1st July 2017. Available online: https://www.finddx.org/wp-content/uploads/2017/03/Ultra-WHO-report_24MAR2017_FINAL.pdf
- Walker TM, Kohl TA, Omar SV, et al. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect Dis 2015;15:1193-202. [Crossref] [PubMed]
- Witney AA, Cosgrove CA, Arnold A, et al. Clinical use of whole genome sequencing for Mycobacterium tuberculosis. BMC Med 2016;14:46. [Crossref] [PubMed]
- Jacobson KR, Theron D, Victor TC, et al. Treatment outcomes of isoniazid-resistance tuberculosis patient, Western Cape Province, South Africa. Clin Infect Dis 2011;53:369-72. [Crossref] [PubMed]
- Warren RM, Streicher EM, Gey van Pittius NC, et al. The clinical relevance of Mycobacterial pharmacogenetics. Tuberculosis (Edinb) 2009;89:199-202. [Crossref] [PubMed]
- Operario DJ, Koeppel AF, Turner SD, et al. Prevalence and extent of heteroresistance by next generation sequencing of multidrug-resistant tuberculosis. PLoS One 2017;12:e0176522. [Crossref] [PubMed]
- Nimmo C, Doyle R, Burgess C, et al. Rapid identification of a Mycobacterium tuberculosis full genetic drug resistance profile through whole genome sequencing directly from sputum. Int J Infect Dis 2017;62:44-6. [Crossref] [PubMed]
- McNerney R, Clark TG, Campino S, et al. Removing the bottleneck in whole genome sequencing of Mycobacterium tuberculosis for rapid drug resistance analysis: a call to action. Int J Infect Dis 2017;56:130-5. [Crossref] [PubMed]
- RESIST-TB. DR-TB clinical trials progress report. Accessed on 1st July 2017. Available online: http://www.resisttb.org/?page_id=1602
- Gibney KB, MacGregor L, Leder K, et al. Vitamin D deficiency is associate with tuberculosis and latent tuberculosis infection in immigrants from Sub-Saharan Africa. Clin Infect Dis 2008;46:443-6. [Crossref] [PubMed]
- Pareek M, Innes J, Sridhar S, et al. Vitamin D deficiency and TB disease phenotype. Thorax 2015;70:1171-80. [Crossref] [PubMed]
- Salahuddin N, Ali F, Hasan Z, et al. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect Dis 2013;13:22. [Crossref] [PubMed]
- Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2009;179:843-50. [Crossref] [PubMed]
- Daley P, Jagannathan V, John KR, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2015;15:528-34. [Crossref] [PubMed]
- Kon OM, Ormerod LP. The national TB strategy: jointly taking responsibility for TB control? Thorax 2015;70:211-2. [Crossref] [PubMed]
- Kumar D, Citron KM, Leese J, et al. Tuberculosis among the homeless at a temporary shelter in London: report of a chest x ray screening programme. J Epidemiol Community Health. 1995;49:629-33. [Crossref] [PubMed]
- Perlman DC, Salomon N, Perkins MP, et al. Tuberculosis in drug users. Clin Infect Dis. 1995;21:1253-64. [Crossref] [PubMed]
- Kumar D, Watson JM, Charlett A, et al. Tuberculosis in England and Wales in 1993: results of a national survey. Public Health Laboratory Service/British Thoracic Society/Department of Health Collaborative Group. Thorax. 1997;52:1060-7. [Crossref] [PubMed]
- Rose AM, Watson JM, Graham C, et al. Tuberculosis at the end of the 20th century in England and Wales: results of a national survey in 1998. Thorax 2001;56:173-9. [Crossref] [PubMed]
- Anderson SR, Maguire H, Carless J. Tuberculosis in London: a decade and a half of no decline Thorax 2007;62:162-7. [corrected]. [Crossref] [PubMed]
- Pai M, Bhaumik S, Bhuyan SS. India's plan to eliminate tuberculosis by 2025: converting rhetoric into reality. BMJ Glob Health 2017;2:e000326. [Crossref] [PubMed]
- Abubakar I, Lipman M, McHugh TD, et al. Uniting to end the TB epidemic: advances in disease control from prevention to better diagnosis and treatment. BMC Med 2016;14:47. [Crossref] [PubMed]
Cite this article as: Kumar K, Kon OM. Diagnosis and treatment of tuberculosis: latest developments and future priorities. Ann Res Hosp 2017;1:37.