
Brain N-acetylaspartate accumulation in Canavan disease is not neurotoxic per se: the implications of the first gene replacement therapy study to demonstrate successful post-symptomatic treatment in mice
Canavan disease (CD) is identified as a fatal spongiform leukodystrophy caused by missense mutations in the Aspa gene (1,2). The latter encodes aspartoacylase (ASPA; EC 3.5.1.15), an enzyme that is highly abundant in oligodendrocytes, and that under physiological conditions hydrolyses N-acetylaspartate (NAA; N-acetyl-L-aspartic acid) to L-aspartate and acetate (Figure 1A). In CD, ASPA cannot degrade NAA and, as a result, NAA builds-up in the central nervous system (CNS) (4) and is also present in very high amounts in the urine of these patients (5). Over the last decades, three major hypotheses have been set forward in an attempt to link the lack of ASPA activity to the neuropathological features (namely, diffuse spongiform white matter degeneration, dysmyelination and intramyelinic oedema) of CD (6,7): (I) the “acetate-lipid-myelin” hypothesis (where the lack of NAA-derived acetate is believed to hinder myelin lipid synthesis) (5,8); (II) the “osmotic-hydrostatic” hypothesis (where NAA accumulation in the CNS is suggested to act as a local water molecule trafficker, leading to the formation of intramyelinic oedema) (9), and (III) the more recent “oxidative stress” hypothesis (where NAA catabolism disruption is believed to be a source of oxidative stress that affects optimal myelination) (10). However, to date, none of these, admittedly non-mutually-exclusive, hypotheses has inspired a clinically effective non-genetic approach to the treatment of CD (6); a fact that has highlighted the need for an efficient gene therapy for this rare and devastating disease.
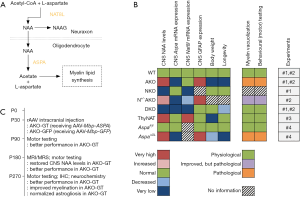
In what appears to be a major study in the field, von Jonquieres et al. (3) have recently shown that although increased CNS NAA levels predict pathological severity in CD mice, these same high levels are not neurotoxic per se. In a series of well-designed experiments using transgenic mice, the authors have managed to show: (I) that the overexpression of the neuronal NAT8L enzyme (N-acetyltransferase-8-like; Figure 1A) that results in high CNS NAA levels, is not linked to CD-simulating neurological deficits; (II) that the targeted, oligodendrocyte-specific elimination of the ASPA enzyme (termed as “conditional ASPA deletion”) is enough to provoke a CD-like pathology on its own, and (III) that the ASPA activity outside the CNS is useful in lowering CNS NAA levels, ameliorating the CD-like pathology and delaying the CD-linked symptomatology onset in transgenic mice with a conditional (oligodendrocyte-specific) Aspa deletion (3). Figure 1B provides a visual synopsis of some of the major findings of four of the in vivo experiments performed by von Jonquieres et al. (3).
In a fifth experiment, von Jonquieres et al. (3) have also shown that 5 and 9 months after the delivery of intracranial myelin basic protein (Mbp)-ASPA gene therapy through an adeno-associated virus (AAV) vector, the Aspa-knockout mice exhibit a post-symptomatic, near-complete regression of their CD-like pathology (Figure 1C). This regression seems to be accompanied by an improved performance in behavioural (motor) tests in the gene therapy-receiving mice as compared to their respective controls; a finding that signifies the potential of this post-symptomatic treatment approach.
The AAV-mediated ASPA delivery in experimental approaches to CD using animals is not a new concept; in fact, several promising attempts have taken place over the last 20 years to produce ASPA in Aspa-knockout or Aspa-mutated rodents (11-14). On this particular occasion, von Jonquieres et al. (3) had a complementary DNA (cDNA) encoding human ASPA been introduced between the mouse Mbp promoter and the woodchuck post-transcriptional regulatory element, followed by a bovine growth hormone poly(A) in an AAV2 plasmid; the latter was packaged in AAV vectors of serotype cy5. This approach was ingenious in that: (I) it allowed for a targeted, oligodendrocyte-specific, AAV-mediated gene therapy based on recent studies that managed to achieve this (15,16), and (II) it embraced a gene therapy approach that has already shown promising results in clinical studies (11,17). However, the recent study of von Jonquieres et al. (3) is not only important for the rationale behind the choice of the employed gene therapy methodology, but also for its translational value, as the gene therapy presented in it is post-symptomatic. The Aspa-knockout mice received their Mbp-ASPA gene therapy at the age of postnatal day 30 (P30; Figure 1C), which is a rather late time-point for murine neurodevelopmental standards, at which these mice already seem to exhibit aspects of a CD-mimicking symptomatology; see supplementary material of (3). In this view, this targeted, post-symptomatic and seemingly effective gene therapy approach signifies a big step forward not only for CD, but also for a number of other rare and devastating leukodystrophies that could benefit from such a cell-type-restricted transgene expression-focused therapeutic approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Matalon R, Michals K, Sebesta D, et al. Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet 1988;29:463-71. [Crossref] [PubMed]
- Hoshino H, Kubota M. Canavan disease: clinical features and recent advances in research. Pediatr Int 2014;56:477-83. [Crossref] [PubMed]
- von Jonquieres G, Spencer ZHT, Rowlands BD, et al. Uncoupling N-acetylaspartate from brain pathology: implications for Canavan disease gene therapy. Acta Neuropathol 2018;135:95-113. [Crossref] [PubMed]
- Janson CG, McPhee SW, Francis J, et al. Natural history of Canavan disease revealed by proton magnetic resonance spectroscopy (1H-MRS) and diffusion-weighted MRI. Neuropediatrics 2006;37:209-21. [Crossref] [PubMed]
- Hagenfeldt L, Bollgren I, Venizelos N. N-acetylaspartic aciduria due to aspartoacylase deficiency--a new aetiology of childhood leukodystrophy. J Inherit Metab Dis 1987;10:135-41. [Crossref] [PubMed]
- Roscoe RB, Elliott C, Zarros A, et al. Non-genetic therapeutic approaches to Canavan disease. J Neurol Sci 2016;366:116-24. [Crossref] [PubMed]
- Baslow MH. Canavan's spongiform leukodystrophy: a clinical anatomy of a genetic metabolic CNS disease. J Mol Neurosci 2000;15:61-9. [Crossref] [PubMed]
- Baslow MH, Guilfoyle DN. Canavan disease, a rare early-onset human spongiform leukodystrophy: insights into its genesis and possible clinical interventions. Biochimie 2013;95:946-56. [Crossref] [PubMed]
- Baslow MH. Brain N-acetylaspartate as a molecular water pump and its role in the etiology of Canavan disease: a mechanistic explanation. J Mol Neurosci 2003;21:185-90. [Crossref] [PubMed]
- Francis JS, Strande L, Markov V, et al. Aspartoacylase supports oxidative energy metabolism during myelination. J Cereb Blood Flow Metab 2012;32:1725-36. [Crossref] [PubMed]
- Leone P, Shera D, McPhee SW, et al. Long-term follow-up after gene therapy for Canavan disease. Sci Transl Med 2012;4:165ra163. [Crossref] [PubMed]
- Matalon R, Surendran S, Rady PL, et al. Adeno-associated virus-mediated aspartoacylase gene transfer to the brain of knockout mouse for Canavan disease. Mol Ther 2003;7:580-7. [Crossref] [PubMed]
- McPhee SW, Francis J, Janson CG, et al. Effects of AAV-2-mediated aspartoacylase gene transfer in the tremor rat model of Canavan disease. Brain Res Mol Brain Res 2005;135:112-21. [Crossref] [PubMed]
- Francis JS, Wojtas I, Markov V, et al. N-acetylaspartate supports the energetic demands of developmental myelination via oligodendroglial aspartoacylase. Neurobiol Dis 2016;96:323-34. [Crossref] [PubMed]
- von Jonquieres G, Fröhlich D, Klugmann CB, et al. Recombinant human myelin-associated glycoprotein promoter drives selective AAV-mediated transgene expression in oligodendrocytes. Front Mol Neurosci 2016;9:13. [Crossref] [PubMed]
- Georgiou E, Sidiropoulou K, Richter J, et al. Gene therapy targeting oligodendrocytes provides therapeutic benefit in a leukodystrophy model. Brain 2017;140:599-616. [PubMed]
- Leone P, Janson CG, Bilaniuk L, et al. Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann Neurol 2000;48:27-38. [Crossref] [PubMed]
Cite this article as: Elliott C, Al-Humadi H, Gutierrez-Quintana R, Zarros A. Brain N-acetylaspartate accumulation in Canavan disease is not neurotoxic per se: the implications of the first gene replacement therapy study to demonstrate successful post-symptomatic treatment in mice. Ann Res Hosp 2018;2:4.


