
Early, short and long-term mortality in community-acquired pneumonia
Introduction
Community-acquired pneumonia (CAP) poses as a health concern for society. Studies have reported that the annual occurrence of CAP ranges from 1.2 to 48 cases per 1,000 inhabitants, being higher in older patients (1,2). However, the incidence in CAP differs by region, season and population characteristics. Moreover, CAP is a frequent reason for hospitalization—typically nearly 20–25% require inpatient treatment. Severe CAP, classified by patient’s admission into the intensive care unit (ICU), develops in about 10–20% of hospitalized patients (3,4). Expenses related to CAP are high. It was estimated that healthcare costs per inpatient admission for CAP ranged from U$11,148 to U$51,219 (5). The overall burden of CAP is expected to increase as the incidence and size of the elderly population grow over coming decades (2).
Mortality associated with CAP remains high, even after excluding patients living in nursing homes or those confined to bed prior to onset of CAP. CAP mortality rate matches with those of other known medical emergency diseases such as ST-elevation myocardial infarction (6). Therefore, it has been stated that CAP should be recognized as a medical emergency due to it being one of the major contemporary acute life-threatening conditions (6,7). However, no effort has been made to organize public health systems in order to reduce short and long-term mortality associated to CAP.
A considerable number of recognizable risk factors may impact CAP mortality. Importantly, associated risk factors and causes of mortality of patients with CAP can vary according to the time in which mortality is evaluated. Factors that may impact mortality within the beginning days could vary from those related to mortality taking place at a later time. According to the available studies, we can classify the timing of mortality of hospitalized patients with CAP as (I) early mortality (generally within the first 48 hours to 7 days after hospital admission); (II) short-term mortality (frequently measured in the first 28–30 days after diagnosis or during hospital admission); and (III) mortality occurring months or years post hospital discharge (long-term mortality). Early mortality has not been evaluated to a great extent as a clinical outcome. However, the majority of studies have centered on short-term adverse events related to CAP. Importantly, studies evaluating short-term mortality also include patients with early mortality. Likewise, throughout the last thirty years, numerous studies have stated variable long-term mortality rates for patients suffering from CAP (8).
In this article, we performed a narrative review about frequency, associated risk factors and causes of early, short and long-term mortality in CAP.
Early mortality (within the first 48 hours to 7 days after hospital admission)
Information about the causes and factors as per mortality within the first 48 hours to 7 days of CAP is scarce. The classic concept is that very early deaths are not as dependent on antimicrobial efficacy as on other factors, including inadequate host response (9-11).
The rate of early mortality has been reported in some CAP studies. Mortensen et al. (11) performed a study, which involved 787 patients from two hospitals. The mean age of CAP patients was 60 years; 79% were male, 84% were admitted through the emergency department, and 20% were admitted to the ICU within the first 24 hours post admission. Mortality stood at 2.5% upon 48-hour mark. Similarly, Garcia-Vidal et al. (9) documented 57 (2.3%) early deaths (<48 hours) among 2,457 hospitalized patients with CAP. Finally, 7 (26.9%) of 26 patients with CAP died within 7 days of admission in another study in which investigators aimed to determine the impact of inflammatory biomarker levels on early mortality (12).
Risk factors associated with early mortality
In a study performed by Bacci et al. (12), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) levels were related with early death, which was defined as death occurring within 7 days of admission. However, C-reactive protein, IL-1 and homocysteine were not associated with this outcome. Moreover, Garcia-Vidal et al. (9) documented that the demographic and clinical aspects of early deaths (<48 hours) describe a group with more severe pneumonia, as shown by the fact that most cases were categorized in the pneumonia severity index (PSI) high severity risk classes. In this study, factors related with early mortality were increased age, altered mental status, multilobar pneumonia, shock upon admission, pneumococcal bacteraemia and discordant empiric antibiotic therapy. This last finding was supported almost exclusively by cases of discordant antimicrobials for patients with P. aeruginosa pneumonia. Altered mental status, multilobar pneumonia, shock upon admission are closely related with an unbalanced inflammatory response to infection.
Although many reports have shown that prescribed empiric antimicrobial regimens are related with reduced mortality at 30 days, there exists a debate about whether appropriate antibiotic selection offers a favorable impact on mortality within the first 48 hours after admission. Mortensen et al. (11) documented in the univariate analysis that variables significantly associated with mortality at 48 hours included living in a nursing home, admission to the ICU within 24 hours, need for mechanical ventilation, altered mental status at onset, arterial hypoxia, and arterial pH 7.35 or less. Furthermore, increasing PSI risk class was associated with increased mortality at 48 hours. After adjusting for potential confounders using the propensity score, use of guideline concordant antibiotics was related with decreased mortality at 48 hours. Table 1 summarizes the factors related with early mortality.

Full table
Causes of early mortality
Causes of early mortality were not reported in most studies (Table 2). Acute respiratory failure (66.6%), septic shock/multiorgan failure (24.6%), congestive heart failure or cardiac arrhythmia (7%), and diabetic ketoacidosis (1.7%) were the causes of early death (<48 hours) documented in non-immunocompromised adults hospitalized with CAP (9). The authors indicated that the great portion of deaths were pneumonia-related, within the environment of an unbalanced inflammatory response.

Full table
Short-term mortality (28 or 30-day or in-hospital mortality)
During the last decades, the short-term prognosis of patients with CAP has been assessed in a great number of studies: a wide range in mortality and differing predictors of mortality were documented (13). Overall short-term mortality rate for CAP patients is dependent on the type of patient in the evaluated cohort. Thus, lower short-term mortality rates have been documented in younger or ambulatory patients, while higher short-term mortality rates in hospitalized or bacteremic patients, and patients requiring ICU admission.
Risk factors associated with short-term mortality
Demographic and clinical features
Fine et al. (13) performed a meta-analysis about the prognosis and outcomes of patients with CAP. Seventeen factors were significantly related with higher risk of mortality (male sex, altered mental status, dyspnea, tachypnea, hypotension, hypothermia, congestive heart failure, alcohol abuse, diabetes mellitus, immunosuppression, neoplastic disease, coronary artery disease, neurologic disease, leukopenia, bacteremia, multilobar radiographic pulmonary infiltrate, and azotemia). However, two factors were related to a lower risk of mortality: chills and pleuritic chest pain. Via multivariate statistical analyses, all of these factors have been recorded as upholding an independent relationship with mortality in one or more individual studies (14-16).
Premorbid poor functional status also has been related with a poor prognostic factor in CAP patients. Functional status has been evaluated by the Eastern Cooperative Oncology Group (ECOG) scale (17), Lawton index (18), Katz index (19) and Barthel index (18,20). Functional status predicted 30-day mortality and improved discrimination and reclassification in consecutive CAP patients (17). Similarly, studies have documented that mortality rate is higher in patients who had acute cardiovascular events (new onset or worsening of cardiac arrhythmias, new onset or worsening of congestive heart failure, stroke and/or myocardial infarction) during an episode of CAP (21-23). Intra-hospital cardiovascular events independently predicted 30-day mortality after adjustment for age, PSI score, and pre-existing comorbid conditions (22,23).
CAP-specific scores
International guidelines recommend varied scores in order to back grading of CAP severity and mortality risk. CURB65 and the PSI are the most widely recommended severity scores. Fine et al. (24) derived a prediction rule (PSI) that classifies patients into five categories as it relates to risk of death within 30 days. The prediction rule assigns points based on age and the presence of coexisting disease, abnormal physical findings and abnormal laboratory findings at presentation. The prediction rule accurately identifies the patients with CAP who are at low risk for death and other adverse events. Similarly, Lim et al. (25) identified prognostic variables using multiple logistic regression with 30-day mortality as the outcome measure. The author developed a score that includes variables of prognostic importance, easily measurable upon initial assessment. A six-point score, one point for each: confusion, urea >7 mmol/L, respiratory rate ≥30 times/min, low systolic (<90 mmHg) or diastolic (≤60 mmHg) blood pressure, age ≥65 years (CURB-65 score). This score allowed for patients to be categorized per rising risk of mortality: from score 0, 0.7% to score 5, 57%. A systematic review and meta-analysis discovered that PSI and CURB-65 predict 30-day mortality in CAP with moderate to good accuracy. No significant differences in overall test performance existed between these scores (26).
Other scores also have been linked with an increased risk of short-term mortality in CAP. CURSI (confusion, urea >7 mmol/L, respiratory rate ≥30 times/min and shock index) (27), quick Sequential (sepsis-related) Organ Failure Assessment (qSOFA) (28), neutrophil-lymphocyte count ratio (29), APACHE II (30), severe CAP (SCAP) score (31), American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) criteria (32), and predisposition, infection/insult, response, organ dysfunction (PIRO) score (33).
Biomarkers
Several humoral and cellular systems that mediate the host response against infection are activated during the acute episode of CAP, so investigators have suggested that such parameters could be of value in the prediction of prognosis in this disease. With such aim, the suitability of biomarkers has been assessed in various CAP studies. Studies have defined the relationship between short-term mortality in CAP and biomarkers of cardiovascular, coagulation, endocrine and immune pathways. However, studies vary significantly in design, setting, sample size and evaluation period. Furthermore, most studies did not control for factors that could affect the accuracy of the biomarkers predicting short-term mortality, such as age, medications and comorbidities (34).
Recently, a systematic review and meta-analysis were performed to investigate the prognostic value of different biomarkers and compare their accuracy as it relates to the established CAP-specific scores (PSI and CURB-65) used for predicting short-term mortality among patients with CAP (35). The authors found that the levels of studied biomarkers were associated with short-term mortality and had moderate to good accuracy in predicting this outcome in CAP. Pro-adrenomedullin, prohormone forms of atrial natriuretic peptide (ANP), cortisol, and procalcitonin had similar accuracy when predicting mortality. However, copeptin and C-reactive protein had the worst predictive performance compared with other biomarkers. However, none of the biomarkers demonstrated a clear advantage over the CAP-specific scores.
Other biomarkers related with increased risk of short-term mortality in CAP were albumin (36,37), cortisol (38), soluble RAGE (39), D-dimer (40), interleukins (41,42) 25-hydroxyvitamin D (43,44), YKL-40, CCL18, SP-D (45), presepsin (46), lactate (47,48) and endothelin-1 (49).
The utility of adding biomarkers to CAP-specific severity scores to predict mortality has also been assessed. Adding biomarkers to scores such as the PSI, CURB-65, APACHE II and SOFA did improve their predictive capability. However, studies are not consistent in relation to these findings. N-terminal pro-brain natriuretic peptide, D-dimer and mid-regional pro-adrenomedullin improved the predictive value for scores in some studies but not in others (50-54). By the same token, studies have assessed the predictive value of combining biomarkers from distinct biological pathways. One multicenter study assessed the prognostic accuracy of five prohormones (adrenomedullin, endothelin-1, ANP, antidiuretic hormone and procalcitonin) (52). Adding all these biomarkers instead of just one (pro-adrenomedullin) led to a significant improvement in the model for CURB-65 but not for PSI. In another study (51), C-reactive protein and IL-6 were independently associated with mortality. The addition of several biomarkers also was found to raise the predictive value of CAP-specific scores.
Causes of short-term mortality
Causes of death within 28 or 30-day after hospital admission or during hospitalization in CAP patients have not been systematically reported (Table 3). Mortensen et al. (55) performed a study to ascertain the causes of death for individuals with CAP. The investigators found pneumonia-related deaths occur more frequently within 30-day after onset. The most frequent immediate causes of death (defined as the disease process, injury, or complication immediately preceding death) for pneumonia-related mortality were respiratory failure, pneumonia, multisystem organ failure and sepsis.

Full table
Other studies have documented that the main causes of 30-day mortality in CAP individuals were respiratory failure and shock/multiorgan failure, followed by acute coronary syndrome, nosocomial infections, sudden death, and stroke (56,57).
Long-term mortality (months or years after hospital discharge)
There is evidence suggesting that hospitalized individuals with CAP have a higher frequency of long-term evidence mortality, even several years after the initial episode. Importantly, decreased long-term survival in CAP patients remained statistically significant even after adjusting for confounding factors (58). Studies have reported long-term mortality rates of 7.2% to 61.9% (59-64). Variability in long-term mortality rate found in the reports was dependent upon several factors such as demographic characteristics, comorbidities, ambulatory vs. hospitalized patient, severity of illness at presentation, and time to follow-up.
Underlying reasons of higher long-term mortality are not clear and there are no specific preventive treatments (65). Authors have hypothesized CAP could affect long-term outcomes via alterations in underlying biological processes (e.g., persistent systemic inflammatory activity), or because CAP could be a marker for physiological compromise or frailty (59). Therefore, it has been noted that additional research is in need in order to determine contributors to long-term mortality in CAP.
A study evaluated long-term survival of hospitalized individuals with CAP in comparison to those hospitalized in a medical ward without CAP. The investigators performed analysis adjusting for age and preexisting comorbidities. The survival resulted significantly lower among CAP patients in comparison with non-CAP patients (60). Similarly, Eurich et al. (59) performed a large, prospective cohort study that made an age, sex-matched comparison of long-term mortality between adult individuals with CAP and patients without pneumonia from the same settings and period. During a median follow-up of 9.8 years, of the 6,078 CAP patients, 2,858 (70 per 1,000 patient years) died during the follow-up period, in comparison to 9,399 (40 per 1,000 patient years) controls.
Another study used database and death certificates to ascertain mortality rates of up to 7 years after discharge (61). In individuals who had demonstrated recovery from CAP, cumulative 1-, 5- and 7-year mortality rates were 17%, 43% and 53%, respectively, in comparison to 4%, 19% and 24%, for an age-matched and sex-matched population reference cohort. Moreover, in the pneumonia patient outcomes research team (PORT) cohort study (mean follow-up duration, 5.9 years) (66), there existed a statistically significant higher mortality rate among patients with CAP across all age groups compared with that of an age-matched cohort for whom data was derived from US life tables. Similarly, in a population-based cohort study of elderly in Finland, patients that survived CAP from 1983 to 1994 were followed up for a median of 9.2 years later (67). The long-term survival rate was significantly lower in people who had survived CAP or pneumococcal CAP than in the rest of study population.
Moreover, a cohort study compared mortality after hospital discharge caused by CAP with mortality of hospitalized patients caused by several other acute and chronic conditions (68). Long-term mortality after hospitalization for CAP was similar to or higher than that associated with after an initial hospitalization for chronic heart failure, cerebrovascular accident, or fracture. One and 5-year mortality rates after an initial hospitalization for CAP were lower than that of subjects initially hospitalized for cancer. These risk estimates remained constant after adjusting for demographic characteristics, smoking status, functional status, nutritional markers, chronic health conditions and circulating concentrations of inflammatory markers.
Risk factors associated with long-term mortality
Demographic and clinical features
Studies have documented age, male sex, CAP severity and nursing home as risk factors related to long-term mortality. In addition, some clinical features upon admission have been related with long-term deaths, while others have been documented as protective factors. Furthermore, etiology of CAP does not seem to be related with long-term mortality.
A study investigated the relationship between clinical parameters and long-term mortality (18 months) in CAP patients. Male sex proved to be a demographic risk factor for long-term mortality. Conversely, initial presentation with temperature >38.7 °C, chills and highest quartile of C-reactive protein were independent protective factors (69). Similarly, Mortensen et al. (66) found that the only “acute” pneumonia-related factors associated with long-term outcomes were feverishness upon presentation (related to reduced mortality) and the presence of pleural effusion (associated with higher mortality). The authors hypothesize that survival of a clinically and biochemically pronounced CAP may reflect a more vigorous host defense as well as a healthier general condition with less complications in the long run.
Another study found (61) that age >65 years, moderate to severe pneumonia CAP (PSI >90) and nursing home residence were independently associated with higher mortality during long-term follow-up. This data concurs with Adamuz et al. (62) results, who also found that nursing homes were independently associated with 1-year mortality, as well as Alan et al. (70), who documented that long-term mortality also was significantly elevated in individuals with higher PSI and CURB-65 scores. Likewise, in a large population-based cohort of patients with pneumonia and up to 5.4 years of follow-up; older age, male sex and higher calculated PSI scores were attributed with higher long-term mortality, with 92 (15%) of PSI class I–II patients dying in comparison to 616 (82%) PSI class V patients (63).
In the pneumonia PORT cohort study, (66) sociodemographic factors tied alongside with long-term mortality were age (stratified by decade), secondary education level or lower, male sex, and nursing home residence. Furthermore, steroid use was independently attributed with mortality. Similarly, Hedlund et al. (71) also found that chronic corticosteroid use was related to a higher risk of mortality in another study. The major reasons for steroid treatment were chronic obstructive pulmonary disease (COPD) as well as connective tissue or joint diseases.
Finally, it seems that etiology of CAP is not related with long-term morality. Ajayi et al. (72) performed a study in adults discharged after an episode of invasive pneumococcal disease. Patients were followed-up throughout three decades. No associations were found between any specific Streptococcus pneumoniae serotype and increased mortality. Similarly, microbial etiology was not able to predict mortality in a 5-year prospective follow-up study (64).
Comorbidities
The presence of comorbidities has been related with long-term mortality in CAP individuals. In the Pneumonia PORT cohort study (66), long-term mortality rate among CAP patients with underlying diseases represented by the Charlson comorbidity score was statistically, significantly more elevated. Similarly, the presence of comorbidity was independently related to higher mortality during long-term follow-up in another study (61), and Ajayi et al. (72) found that the number of comorbid diseases suffered by each adult released post an episode of invasive pneumococcal CAP was related with this outcome.
However, specific comorbid diseases have been evaluated in its influence on long-term mortality. Of the 13 comorbid diseases analyzed in a study (72), cancer and neurologic diseases were the significant variables associated with long-term mortality. Likewise, Guertler et al. (69) found that comorbidities independently associated with 18-month mortality in CAP patients were COPD and neoplastic disease. Similarly, after adjustment for confounders, COPD, diabetes mellitus, cancer, and dementia were independently associated with 1-year mortality in CAP individuals in another study (62). Koskela et al. (73) also saw that a previous diagnosis of diabetes and new postprandial hyperglycaemia among the non-diabetic population demonstrated independent associations with late mortality.
Holter et al. (64) found that underlying diseases related with late death were cardiovascular disease, COPD, and immunocompromising. Similarly, Saldías et al. (74) also found that chronic cardiovascular disease was related with this outcome.
Biomarkers
There is growing literature on the utility of biomarkers in predicting long-term outcomes on CAP. There are several biomarkers that reflect different pathophysiological aspects of CAP that have been associated with long-term mortality.
Alan et al. (70) investigated the potential of varying blood biomarkers for long-term mortality prediction in a large and well-defined cohort of CAP individuals from a multicenter study over a 6-year follow-up period. Mortality was increased among patients in the highest pro-adrenomedullin, pro-ANP and urea quartiles. Observations of variations in mortality could not be made between patients in the varying quartiles of C-reactive protein and procalcitonin concentrations and leukocyte count. Similarly, after adjusting for comorbidity and pneumonia severity, Krüger et al. (75) found that mid-regional pro-ANP and copeptin were independent, as well as the strongest predictors of long-term mortality. In another study, N-terminal pro-B-type natriuretic peptide was an independent mortality predictor (76). Moreover, in patients with CAP who survived a 180-day follow-up, mid-regional pro-adrenomedullin, mid-regional pro-ANP, copeptin, and C-terminal proendothelin-1 levels upon admission were significantly lower in comparison to those in patients who passed away. It was also true for the inflammatory marker, procalcitonin, and the CURB-65 score; however, it was not the case for C-reactive protein and leukocytes (77). Vazquez et al. (78) also confirmed the high prognostic performance of mid-regional pro-ANP for long-term mortality.
Elevated high-sensitivity cardiac troponin during acute CAP episode was found to be a predictor of long-term (1–4.1 years) mortality (79). Furthermore, in a secondary follow-up analysis of data from a prospectively recruited well-defined cohort of 241 hospital survivors of CAP, Holter et al. (65) witnessed a statistically significant association between vitamin D deficiency upon hospital admission and long-term, all-cause mortality after discharge in these patients. The patterns of TNF, IL-6, IL-10, D-dimer, antithrombin-III, and factor IX witnessed among men could be associated with poorer long-term survival rates in another study (80).
Finally, elevations of thrombin-antithrombin III-complexes and D-dimer levels were documented as common at the moment of discharging individuals who gave the impression to have improved clinically from CAP. Thrombin-antithrombin III-complexes and D-dimer levels were related with increased risks of subsequent deaths, particularly as result of cardiovascular disease (81). In this study, patients who overcome the initial episode of CAP present with persistent inflammation or coagulation activation upon hospital discharge; this inflammation correlates with higher mortality post discharge. Investigators suggested that an acute infectious process, albeit resolved, creates tenacious alterations, accelerating the advancement of baseline comorbidities that induce earlier mortality.
However, care must be taken when interpreting cardiovascular biomarkers as risk factors associated for long-term mortality. The elevation of these biomarkers during an acute episode of CAP could be the result of underlying, pre-existing cardiac disease or septic cardiomyopathy. As a result, due to acute inflammatory activation, CAP may aggravate an underlying cardiovascular disease, which could or could not be known before admission.
Others
Another study evaluated whether the development of intra-hospital cardiac complications may impact mortality and cardiovascular events taking place throughout a long-term follow up of CAP patients post-hospital discharge (82). In the follow-up, 89 patients passed away (51% of whom had an intra-hospital cardiac complication and 26%, without). A Cox regression analysis illustrated that intra-hospital cardiac complications, age and PSI were significantly associated to general long-term mortality.
Moreover, Koskela et al. (73) evaluated 153 consecutive hospitalized individuals who remained alive at least 30 days post mild-to-moderate CAP. The surveillance status was recorded after a median of 5 years and 11 months. In that study, Karnofsky score ≤80% increases the risk of death for several years after CAP. Other studies have documented that low serum albumin levels upon hospital admission are associated with long-term mortality in CAP (64). Table 4 shows the factors related to long-term mortality in CAP.
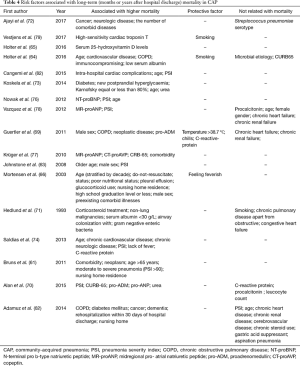
Full table
Causes of long-term mortality
Causes of long-term mortality in CAP individuals have been reported in some studies (Table 5). Holter et al. (64) documented that chronic diseases, including COPD, vascular diseases and malignancy were primary reasons for long-term mortality in CAP patients followed-up for 5 years after hospital discharge. The occurrence of vascular deaths was at its peak within the first-year post CAP, while deaths from COPD and malignancies took place at a more constant level during follow-up. Similarly, Adamuz et al. (62) evaluated causes of mortality in CAP patients followed-up for 1 year following hospital discharge. They documented that the main reasons were infectious diseases, primarily pneumonia, succeeded by acute cardiovascular events. Mortality from infectious diseases was more elevated throughout the initial 6 months, while the number of deaths from cardiovascular causes remained constant during follow-up months. In another study, the primary underlying reasons for late death in CAP individuals were cardiovascular, obstructive lung disease and cancer. In this study, miscellaneous causes accounted for 25% of all cases (73).
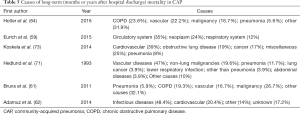
Full table
Moreover, other studies have compared causes of long-term mortality occurring in CAP individuals with those in controls. Eurich et al. (59) documented that the three most common causes of death after hospital discharge in CAP patients were circulatory system, neoplasm, and respiratory system. Cause of death was alike for both CAP patients and controls, with the exception of respiratory causes which were significantly more frequent in CAP individual (24% vs. 9% for controls, P<0.001). Moreover, Bruns et al. (61) found that malignancy (27%), COPD (19%) and cardiovascular disease (16%) were the most frequent causes of death. Interestingly, only 6% passed away of pneumonia, in comparison to 3.2% within the general population. Patients who pulled through an episode of CAP had an approximately fourfold increased risk of having COPD recorded as their cause of death in comparison to the general population (19.3% vs. 4.4%). Inversely, general population controls presented a more elevated risk of death from cardiovascular diseases than the CAP population (30.2% vs. 16.0%). Also, 5.9% of patients who had experienced a prior episode of CAP died of recurrent pneumonia, in comparison to a 3.2% risk of death from pneumonia in the general population.
Conclusions
CAP continues to be related to high morbidity, mortality, and health costs. The associated risk factors and causes of mortality of individuals presenting with CAP vary according to the time in which mortality is evaluated. Information about the causes and factors related to mortality within the first 48 hours to 7 days of CAP is scarce. Studies suggest that early deaths depend on severity of the disease upon admission as well as antimicrobial efficacy. Moreover, short-term mortality in CAP has been widely evaluated. Age and the presence of coexisting diseases, abnormal physical findings and abnormal laboratory findings upon presentation have been associated with short-term mortality. Finally, although information regarding long-term mortality in CAP is growing, underlying reasons of higher long-term mortality are not clear and there are no specific preventive treatments. Multiple risk factors that include age, sex, comorbid conditions and persistence of inflammation upon hospital discharge are attributed with higher long-term mortality. However, most causes of death are related with newly diagnosed or underlying chronic diseases. Unlike short-term mortality, the severity of the physiologic abnormalities at initial presentation are not significantly related with an elevated long-term mortality. What remains controversial is whether CAP is a causal risk factor or a risk marker for an underlying, and possibly unrecognized process that increases the risk for late death. Moreover, several biomarkers have shown to be independently related to short and long-term mortality.
In spite of progress made regarding the understanding of mortality among CAP patients, there remains an unacceptably elevated mortality rate. Due to the complexity factors that could impact CAP mortality, studies in the future would best set forth reliable strategies for improving outcomes in patients with CAP.
Acknowledgements
We would thank Anthony Armenta Jr for the paper’s English edition.
Funding: C Garcia-Vidal has received the INTENSIFICACIÓ grant from the Strategic Plan for Research and Innovation in Health (PERIS) 2016–2020.
Footnote
Conflicts of Interest: C Garcia-Vidal, P Puerta and CG Cardozo belong to the Fungi CLINIC AGAUR group. The other authors have no conflicts of interest to declare.
References
- Torres A, Peetermans WE, Viegi G, et al. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013;68:1057-65. [Crossref] [PubMed]
- Viasus D, Núñez-Ramos JA, Viloria SA, et al. Pharmacotherapy for community-acquired pneumonia in the elderly. Expert Opin Pharmacother 2017;18:957-64. [Crossref] [PubMed]
- Jain S, Self WH, Wunderink RG, et al. CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415-27. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27-72. [Crossref] [PubMed]
- Sato R, Gomez Rey G, Nelson S, et al. Community-acquired pneumonia episode costs by age and risk in commercially insured US adults aged ≥50 years. Appl Health Econ Health Policy 2013;11:251-8. [Crossref] [PubMed]
- Ewig S, Torres A. Community-acquired pneumonia as an emergency: time for an aggressive intervention to lower mortality. Eur Respir J 2011;38:253-60. [Crossref] [PubMed]
- Kolditz M, Braeken D, Ewig S, et al. Severity Assessment and the Immediate and Long-Term Prognosis in Community-Acquired Pneumonia. Semin Respir Crit Care Med 2016;37:886-96. [Crossref] [PubMed]
- Restrepo MI, Faverio P, Anzueto A. Long-term prognosis in community-acquired pneumonia. Curr Opin Infect Dis 2013;26:151-8. [Crossref] [PubMed]
- Garcia-Vidal C, Fernández-Sabé N, Carratalà J, et al. Early mortality in patients with community-acquired pneumonia: causes and risk factors. Eur Respir J 2008;32:733-9. [Crossref] [PubMed]
- Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med 1964;60:759-76. [Crossref] [PubMed]
- Mortensen EM, Restrepo MI, Anzueto A, et al. Antibiotic therapy and 48-hour mortality for patients with pneumonia. Am J Med 2006;119:859-64. [Crossref] [PubMed]
- Bacci MR, Leme RC, Zing NP, et al. IL-6 and TNF-α serum levels are associated with early death in community-acquired pneumonia patients. Braz J Med Biol Res 2015;48:427-32. [Crossref] [PubMed]
- Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996;275:134-41. [Crossref] [PubMed]
- Simonetti AF, Garcia-Vidal C, Viasus D, et al. Declining mortality among hospitalized patients with community-acquired pneumonia. Clin Microbiol Infect 2016;22:567.e1-7. [Crossref] [PubMed]
- Tashiro M, Fushimi K, Takazono T, et al. A mortality prediction rule for non-elderly patients with community-acquired pneumonia. BMC Pulm Med 2016;16:39. [Crossref] [PubMed]
- Houston MS, Silverstein MD, Suman VJ. Risk factors for 30-day mortality in elderly patients with lower respiratory tract infection. Community-based study. Arch Intern Med 1997;157:2190-5. [Crossref] [PubMed]
- Jeon K, Yoo H, Jeong BH, et al. Functional status and mortality prediction in community-acquired pneumonia. Respirology 2017;22:1400-6. [Crossref] [PubMed]
- Calle A, Márquez MA, Arellano M, et al. Geriatric assessment and prognostic factors of mortality in very elderly patients with community-acquired pneumonia. Arch Bronconeumol 2014;50:429-34. [PubMed]
- Kosai K, Izumikawa K, Imamura Y, et al. Importance of functional assessment in the management of community-acquired and healthcare-associated pneumonia. Intern Med 2014;53:1613-20. [Crossref] [PubMed]
- Murcia J, Llorens P, Sánchez-Payá J, et al. Functional status determined by Barthel Index predicts community acquired pneumonia mortality in general population. J Infect 2010;61:458-64. [Crossref] [PubMed]
- Viasus D, Garcia-Vidal C, Manresa F, et al. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J Infect 2013;66:27-33. [Crossref] [PubMed]
- Corrales-Medina VF, Musher DM, Wells GA, et al. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation 2012;125:773-81. [Crossref] [PubMed]
- Violi F, Cangemi R, Falcone M, et al. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin Infect Dis 2017;64:1486-93. [Crossref] [PubMed]
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243-50. [Crossref] [PubMed]
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377-82. [Crossref] [PubMed]
- Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010;65:878-83. [Crossref] [PubMed]
- Nüllmann H, Pflug MA, Wesemann T, et al. External validation of the CURSI criteria (confusion, urea, respiratory rate and shock index) in adults hospitalised for community-acquired pneumonia. BMC Infect Dis 2014;14:39. [Crossref] [PubMed]
- Ranzani OT, Prina E, Menéndez R, et al. New Sepsis Definition (Sepsis-3) and Community-acquired Pneumonia Mortality. A Validation and Clinical Decision-Making Study. Am J Respir Crit Care Med 2017;196:1287-97. [Crossref] [PubMed]
- de Jager CP, Wever PC, Gemen EF, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One 2012;7:e46561. [Crossref] [PubMed]
- Zhuang Y, Li W, Wang H, et al. Predicting the Outcomes of Subjects With Severe Community-Acquired Pneumonia Using Monocyte Human Leukocyte Antigen-DR. Respir Care 2015;60:1635-42. [Crossref] [PubMed]
- España PP, Capelastegui A, Quintana JM, et al. Validation and comparison of SCAP as a predictive score for identifying low-risk patients in community-acquired pneumonia. J Infect 2010;60:106-13. [Crossref] [PubMed]
- Sibila O, Mortensen EM, Redrow G, et al. Evaluation of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Hosp Pract 1995;2012:158-64. [PubMed]
- Rello J, Rodriguez A, Lisboa T, et al. PIRO score for community-acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit Care Med 2009;37:456-62. [Crossref] [PubMed]
- Viasus D, Simonetti A, Garcia-Vidal C, et al. Prediction of prognosis by markers in community-acquired pneumonia. Expert Rev Anti Infect Ther 2013;11:917-29. [Crossref] [PubMed]
- Viasus D, Del Rio-Pertuz G, Simonetti AF, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: A systematic review and meta-analysis. J Infect 2016;72:273-82. [Crossref] [PubMed]
- Viasus D, Garcia-Vidal C, Simonetti A, et al. Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia. J Infect 2013;66:415-23. [Crossref] [PubMed]
- Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008;47:375-84. [Crossref] [PubMed]
- Kolditz M, Höffken G, Martus P, et al. Serum cortisol predicts death and critical disease independently of CRB-65 score in community-acquired pneumonia: a prospective observational cohort study. BMC Infect Dis 2012;12:90. [Crossref] [PubMed]
- Narvaez-Rivera RM, Rendon A, Salinas-Carmona MC, et al. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC Infect Dis 2012;12:15. [Crossref] [PubMed]
- Chalmers JD, Singanayagam A, Scally C, et al. Admission D-dimer can identify low-risk patients with community-acquired pneumonia. Ann Emerg Med 2009;53:633-8. [Crossref] [PubMed]
- Andrijevic I, Matijasevic J, Andrijevic L, et al. Interleukin-6 and procalcitonin as biomarkers in mortality prediction of hospitalized patients with community acquired pneumonia. Ann Thorac Med 2014;9:162-7. [Crossref] [PubMed]
- Lee YL, Chen W, Chen LY, et al. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. J Crit Care 2010;25:176.e7-13. [Crossref] [PubMed]
- Remmelts HH, van de Garde EM, Meijvis SC, et al. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis 2012;55:1488-94. [Crossref] [PubMed]
- Kim HJ, Jang JG, Hong KS, et al. Relationship between serum vitamin D concentrations and clinical outcome of community-acquired pneumonia. Int J Tuberc Lung Dis 2015;19:729-34. [Crossref] [PubMed]
- Spoorenberg SM, Vestjens SM, Rijkers GT, et al. YKL-40, CCL18 and SP-D predict mortality in patients hospitalized with community-acquired pneumonia. Respirology 2017;22:542-50. [Crossref] [PubMed]
- Liu B, Yin Q, Chen YX, et al. Role of Presepsin (sCD14-ST) and the CURB65 scoring system in predicting severity and outcome of community-acquired pneumonia in an emergency department. Respir Med 2014;108:1204-13. [Crossref] [PubMed]
- Frenzen FS, Kutschan U, Meiswinkel N, et al. Admission lactate predicts poor prognosis independently of the CRB/CURB-65 scores in community-acquired pneumonia. Clin Microbiol Infect 2018;24:306.e1-6. [Crossref] [PubMed]
- Jo S, Jeong T, Lee JB, et al. Validation of modified early warning score using serum lactate level in community-acquired pneumonia patients. The National Early Warning Score-Lactate score. Am J Emerg Med 2016;34:536-41. [Crossref] [PubMed]
- Schuetz P, Stolz D, Mueller B, et al. Endothelin-1 precursor peptides correlate with severity of disease and outcome in patients with community acquired pneumonia. BMC Infect Dis 2008;8:22. [Crossref] [PubMed]
- Jeong KY, Kim K, Kim TY, et al. Prognostic value of N-terminal pro-brain natriuretic peptide in hospitalised patients with community-acquired pneumonia. Emerg Med J 2011;28:122-7. [Crossref] [PubMed]
- Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax 2009;64:587-91. [Crossref] [PubMed]
- Schuetz P, Wolbers M, Christ-Crain M, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care 2010;14:R106. [Crossref] [PubMed]
- Suberviola B, Castellanos-Ortega A, Llorca J, et al. Prognostic value of proadrenomedullin in severe sepsis and septic shock patients with community-acquired pneumonia. Swiss Med Wkly 2012;142:w13542. [PubMed]
- Snijders D, Schoorl M, Schoorl M, et al. D-dimer levels in assessing severity and clinical outcome in patients with community-acquired pneumonia. A secondary analysis of a randomised clinical trial. Eur J Intern Med 2012;23:436-41. [Crossref] [PubMed]
- Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med 2002;162:1059-64. [Crossref] [PubMed]
- Viasus D, Garcia-Vidal C, Castellote J, et al. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore) 2011;90:110-8. [Crossref] [PubMed]
- Chang CL, Mills GD, Karalus NC, et al. Biomarkers of cardiac dysfunction and mortality from community-acquired pneumonia in adults. PLoS One 2013;8:e62612. [Crossref] [PubMed]
- Peyrani P, Ramirez J. Long-term Mortality in Hospitalized Patients With Community-Acquired Pneumonia. Am J Med Sci 2017;353:421. [Crossref] [PubMed]
- Eurich DT, Marrie TJ, Minhas-Sandhu JK, et al. Ten-Year Mortality after Community-acquired Pneumonia. A Prospective Cohort. Am J Respir Crit Care Med 2015;192:597-604. [Crossref] [PubMed]
- Bordon J, Wiemken T, Peyrani P, et al. Decrease in long-term survival for hospitalized patients with community-acquired pneumonia. Chest 2010;138:279-83. [Crossref] [PubMed]
- Bruns AH, Oosterheert JJ, Cucciolillo MC, et al. Cause-specific long-term mortality rates in patients recovered from community-acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect 2011;17:763-8. [Crossref] [PubMed]
- Adamuz J, Viasus D, Jiménez-Martínez E, et al. Incidence, timing and risk factors associated with 1-year mortality after hospitalization for community-acquired pneumonia. J Infect 2014;68:534-41. [Crossref] [PubMed]
- Johnstone J, Eurich DT, Majumdar SR, et al. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine (Baltimore) 2008;87:329-34. [Crossref] [PubMed]
- Holter JC, Ueland T, Jenum PA, et al. Risk Factors for Long-Term Mortality after Hospitalization for Community-Acquired Pneumonia: A 5-Year Prospective Follow-Up Study. PLoS One 2016;11:e0148741. [Crossref] [PubMed]
- Holter JC, Ueland T, Norseth J, et al. Vitamin D Status and Long-Term Mortality in Community-Acquired Pneumonia: Secondary Data Analysis from a Prospective Cohort. PLoS One 2016;11:e0158536. [Crossref] [PubMed]
- Mortensen EM, Kapoor WN, Chang CC, et al. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis 2003;37:1617-24. [Crossref] [PubMed]
- Koivula I, Stén M, Mäkelä PH. Prognosis after community-acquired pneumonia in the elderly: a population-based 12-year follow-up study. Arch Intern Med 1999;159:1550-5. [Crossref] [PubMed]
- Yende S, Angus DC, Ali IS, et al. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc 2007;55:518-25. [Crossref] [PubMed]
- Guertler C, Wirz B, Christ-Crain M, et al. Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J 2011;37:1439-46. [Crossref] [PubMed]
- Alan M, Grolimund E, Kutz A, et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6-year prospective follow-up study. J Intern Med 2015;278:174-84. [Crossref] [PubMed]
- Hedlund JU, Ortqvist AB, Kalin ME, et al. Factors of importance for the long-term prognosis after hospital treated pneumonia. Thorax 1993;48:785-9. [Crossref] [PubMed]
- Ajayi OO, Norton NB, Gress TW, et al. Three Decades of Follow-up of Adults After Recovery from Invasive Pneumococcal Pneumonia. Am J Med Sci 2017;353:445-51. [Crossref] [PubMed]
- Koskela HO, Salonen PH, Romppanen J, et al. Long-term mortality after community-acquired pneumonia--impacts of diabetes and newly discovered hyperglycaemia: a prospective, observational cohort study. BMJ Open 2014;4:e005715. [Crossref] [PubMed]
- Saldías PF, Maturana OR, Román OF, et al. Long-term survival of immunocompetent patients older than 60 years hospitalized for community-acquired pneumonia. Rev Med Chil 2013;141:831-43. [PubMed]
- Krüger S, Ewig S, Kunde J, et al. Pro-atrial natriuretic peptide and pro-vasopressin for predicting short-term and long-term survival in community-acquired pneumonia: results from the German Competence Network CAPNETZ. Thorax 2010;65:208-14. [Crossref] [PubMed]
- Nowak A, Breidthardt T, Christ-Crain M, et al. Direct comparison of three natriuretic peptides for prediction of short- and long-term mortality in patients with community-acquired pneumonia. Chest 2012;141:974-82. [Crossref] [PubMed]
- Krüger S, Ewig S, Giersdorf S, et al. Cardiovascular and inflammatory biomarkers to predict short- and long-term survival in community-acquired pneumonia: Results from the German Competence Network, CAPNETZ. Am J Respir Crit Care Med 2010;182:1426-34. [Crossref] [PubMed]
- Vazquez M, Jockers K, Christ-Crain M, et al. MR-pro-atrial natriuretic peptide (MR-proANP) predicts short- and long-term outcomes in respiratory tract infections: a prospective validation study. Int J Cardiol 2012;156:16-23. [Crossref] [PubMed]
- Vestjens SM, Spoorenberg SM, Rijkers GT, et al. High-sensitivity cardiac troponin T predicts mortality after hospitalization for community-acquired pneumonia. Respirology 2017;22:1000-6. [Crossref] [PubMed]
- Reade MC, Yende S, D’Angelo G, et al. Differences in immune response may explain lower survival among older men with pneumonia. Crit Care Med 2009;37:1655-62. [Crossref] [PubMed]
- Yende S, D’Angelo G, Mayr F, et al. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS One 2011;6:e22847. [Crossref] [PubMed]
- Cangemi R, Calvieri C, Falcone M, et al. Relation of Cardiac Complications in the Early Phase of Community-Acquired Pneumonia to Long-Term Mortality and Cardiovascular Events. Am J Cardiol 2015;116:647-51. [Crossref] [PubMed]
Cite this article as: Viasus D, Cillóniz C, Cardozo CG, Puerta P, Garavito A, Torres A, Garcia-Vidal C. Early, short and long-term mortality in community-acquired pneumonia. Ann Res Hosp 2018;2:5.


