
Treatment guidelines for community-acquired pneumonia
Introduction
The first guideline for community-acquired pneumonia (CAP) in North American was published in 1993 when treatment of pneumonia was heterogeneous and the individual providers’ approach often superseded an organized synthesis of the scientific literature and a recommended approach to management. Since the first guidelines were released, the number of published articles mentioning pneumonia, recommendations, and guidelines has increased exponentially (1). This reflects recent trends towards guideline-driven recommendations for many common illnesses, along with the observation that many countries prefer national recommendations adapted to their own healthcare environment. This review focuses on the continued utility of CAP guidelines, providing an overview of the treatment principles supported by guidelines.
Why CAP guidelines remain useful
The continued relevance of CAP guidelines stems from numerous worldwide studies showing improved outcomes including lower in-hospital mortality and days intubated, fewer patients requiring mechanical ventilation, along with reduced costs and length of stay, when guideline-based treatment approaches were implemented. (2-6). In the United States, consistent adoption and application of guidelines occurred after the nation’s largest insurer, Medicare, developed specific performance measures for antibiotic therapy of CAP, and began to monitor adherence to these recommendations.
Some of the strongest evidence supporting the application of guideline-based therapy exists in the treatment of severe CAP, where adherence is associated with reduced mortality (7,8). Retrospective studies also suggest that application of CAP guidelines improves outcomes in patients with non-severe CAP, with one large study of non-intensive care unit (ICU) hospitalized CAP patients showing that while 65% of patients received guideline concordant therapy, after adjusting for confounders, concordant therapy was associated with decreased in-hospital mortality, sepsis, and renal failure as well as reduced length of stay and duration of intravenous antibiotic therapy (9). Despite this evidence, and consistent with other clinical practice guidelines, CAP treatment guidelines have been controversial and are not uniformly followed (Table 1) (10). Explanations for this paradox include physician preference to tailor antibiotic therapy to specific patient risk factors including prior resistant infections, individual patient’s antimicrobial tolerance, and the severity of underlying acute and chronic illness.

Full table
Overview of global CAP guidelines
Specific CAP guidelines exist throughout the globe and, high-quality and updated guidelines, tailored to the regional, national, or local realities are necessary as one international guideline is not sufficient to meet all the demands of each practice region. The major determinants driving the need for regional instead of global guidelines, result from differences in socioeconomic factors, health care systems, local criteria regarding the need for hospitalization, and variations in antimicrobial availability. Surprisingly, or not, the differences between regions in regards to the possible pathogenic microorganisms, with the exception of areas with a high prevalence of tuberculosis presenting as CAP, do little to explain the idiosyncrasies contained amongst alternative recommendations. For instance, one review compared the etiology of CAP in different studies from Spain, Japan, Italy, the Netherlands, and the United States, and consistently found Streptococcus pneumoniae to be the most common causative pathogen, followed by Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophilia, and Hemophilus influenzae, respectively, regardless of world region (11). Another study using a comprehensive and systematic approach to identify CAP pathogens in the United States, Europe, Latin America, and the Asia-Pacific area, found little worldwide difference in the microbiology associated with CAP, but with differences in therapy choices, that in turn, correlated with mortality (12). One important area where the differences between regional guidelines become clearly relevant is when consideration is given to differences in socioeconomic aspects, such as malnutrition and the incidence of HIV/AIDS infection. Both are known to affect CAP etiology. Pneumonia is the second or third leading cause of death among 10 countries with the highest HIV/AIDS prevalence, and the World Health Organization defines most of these countries as low-income (per capita income less than $200 per day). In contrast, pneumonia is the fifth leading cause of death amongst those countries with the highest per capita incomes (13). In the countries with an increased incidence of HIV/AIDS, Pneumocystis jirovecii and other opportunistic infections associated with AIDS tend to make up a larger share of the microorganism burden associated with pneumonia (14). The high prevalence of melioidosis is a unique pneumonia challenge encountered throughout Southeast Asia and Thailand, driving guideline recommendations in these areas, but is rarely seen in other parts of the world.
While regional CAP guidelines have been developed to address unique country and region-specific issues in relation to bacteriology, health policy, and economics, the data reviewed by expert committees to formulate guidelines tends to be similar. The differences that then exist between guidelines can be in part attributed to varying interpretation of the data depending on the background, experience, philosophy, and specialty of the experts designing the guidelines. For instance, both the 2005 European Respiratory Society guideline and the 2007 IDSA/ATS CAP guidelines cited the same study findings about the efficacy of combining a β-lactam and a macrolide or the use of fluoroquinolone monotherapy (15), but came to different conclusions. The European guideline (16) concluded that “the use of such antimicrobial agents in these patients remains very limited” while the American guideline (17) recommendations in favor of these regimens “were based on retrospective studies demonstrating a significant reduction in mortality.”
The 2007 IDSA/ATS guidelines were designed to account for unique epidemiologic challenges along with bacteriologic considerations that may not be relevant in other parts of the world. Also unique to the North American guidelines is incorporation of quality and performance metrics that are specific to the US healthcare system. Important core measures that were collected and reported for every hospital included timely administration of antibiotics, and selection of guideline-recommended antibiotics in the hospital and ICU.
Another difference in the US approach to pneumonia is the recognition of the concept of healthcare associated pneumonia (HCAP), in large part due to the multitude of American patients residing in nursing homes. The presence of HCAP has been used to account for a presumed higher frequency of infections with nosocomial and drug-resistant pathogens in these patients, who by definition are exposed to the healthcare environment (dialysis, home infusion therapy, repeated hospitalizations) (18). Several studies have questioned whether HCAP is a form of CAP or nosocomial pneumonia, but regardless, this entity is an important American concern, while some European experts favor treating these patients as CAP, and one recent European guideline went so far as to state that HCAP is not relevant in Europe (19).
Therapy recommendations for specific pathogens
Important bacteriologic considerations relevant in the United States result in specific recommendations pertaining to the treatment of certain pathogens.
Atypical pathogens
US guidelines recommend that all patients receive empiric therapy for L.pneumophila, C.pneumoniae, and M.pneumoniae. Although there are data to suggest that atypical pathogens occur with similar frequency outside of the US (12), their role is thought to be less important in European guidelines and in recommendations from the British Thoracic Society (20). The American approach is based on studies that illustrate not only a high frequency of atypical pathogens among both outpatients and inpatients, but also retrospective data from severely ill patients, showing that the addition of a macrolide to a β-lactam is associated with improved mortality (21-26). Of note, the benefit of combining a macrolide with beta-lactam appeared to be greater than the addition of a fluoroquinolone to a beta-lactam in several studies and in a meta-analysis (23,26).
While the benefits of macrolide therapy seem more clear in those with severe pneumonia, the use of beta-lactam monotherapy may be as good as a beta-lactam/macrolide combination in outpatients and in admitted patients without serious illness. However, the benefit may be related to the anti-inflammatory benefits of these drugs, and not necessarily to the coverage of atypical pathogens. Worldwide, the mortality rate from CAP parallels the use of macrolide therapy, and not the frequency of atypical pathogens (12).
Pneumococcus
US guidelines recommend the use of a β-lactam plus macrolide regimen, or alternatively, monotherapy with a fluoroquinolone for outpatients and inpatients with comorbid illness or risk factors for drug-resistant streptococcus pneumonia (DRSP). Risk factors for DRSP include: age >65 years, beta-lactam therapy in the past 3 months, alcoholism, immune suppression (including corticosteroid therapy), multiple medical comorbidities, and exposure to a child in daycare. Monotherapy with a macrolide is reserved for selected outpatients and inpatients that are young have no cardiopulmonary comorbidities, no recent antibiotic therapy and no recent hospitalization. Although nearly 40% of pneumococci may be penicillin-resistant in vitro, resistance levels remain low in the US, and resistance typically does not result in adverse clinical outcomes (27). In fact, most penicillin-resistant pneumococci are classified as “intermediate” based on updated US definitions for non-meningeal infection, with minimum inhibitory concentration (MIC) values up to 4 mg/L (resistance is defined as MIC ≥8 mg/L). Accordingly, over time the number of patients with DRSP infections has decreased, with the application of these new definitions. However, infection with invasive resistant-pneumococci, although now less frequently observed, still affects outcomes. In one large study in the US, mortality was increased among patients infected with invasive pneumococcal strains with penicillin MIC ≥4 mg/L. who survived the initial 4 days of hospitalization (27).
While pneumococcal resistance to fluoroquinolones is uncommon, repeated use of a given agent is a risk factor for the development of future resistance and this holds true not only for fluoroquinolones, but also for pneumococcal resistance to β-lactams and macrolides in patients receiving the same antibiotic class within the past 3 months (28). US guidelines reflect this observation, recommending that CAP patients receive an alternative therapy than the prior antibiotics that were given within the past 3 months. Special attention must be given to the empiric use of fluoroquinolones in parts of the world and in patients where the incidence of tuberculosis (TB) is high, as therapy may mask the diagnosis of tuberculosis. It may be prudent to avoid empiric fluoroquinolone therapy in patients originating or traveling from TB endemic areas and in patients with HIV, but more recent data from Taiwan show that when fluoroquinolones were used to empirically to treat severe CAP, that eventually turned out to be undiagnosed tuberculosis, mortality was improved, and there was no delay in starting tuberculosis treatment in those receiving empiric fluoroquinolones (29).
Differences in macrolide resistance patterns may explain why US guidelines recommend this class for CAP treatment regimens in contrast to European guidelines. In Europe, macrolide resistance is due to the inability of the antibiotic to bind to its ribosomal site of action (high level resistance), while macrolide resistance encountered in the US is usually mediated through efflux mechanisms (a lower level of resistance). As a result, macrolide resistance may be less clinically relevant in the US than in Europe, and may be overcome by high local macrolide concentrations at respiratory sites of infection resulting in adequate therapy. However, despite the recommendation that some US patients may receive macrolide monotherapy, few patients in fact receive this treatment approach in the hospital, although it is commonly used in outpatients. Finally, in cases of pneumococcal bacteremia, combination therapy of a β-lactam plus a macrolide, has been shown in multiple studies to reduce mortality when compared with single agent therapy, especially in cases of severe illness, further limiting recommendations for focused beta-lactam monotherapy, even with sensitive pathogens (21,22,26,30,31).
Community-acquired methicillin-resistant staphylococcus aureus (CA-MRSA)
US guidelines do not recommend routine empiric therapy for CA-MRSA, even for those admitted to the ICU. However, guidelines do recommend considering infection with this organism, a pathogen more common in the US than in Europe, in patients with severe CAP and a compatible clinical picture that may include previously healthy individuals, recent viral or influenza infection, associated rash, and severe, bilateral, necrotizing pneumonia. Non-severe presentation of CA-MRSA can also occur (32). When suspicion is high for CA-MRSA, therapy may need to involve both an antibacterial agent and an antitoxin-producing agent, such as vancomycin plus clindamycin or, alternatively linezolid alone, as certain strains of CA-MRSA produce virulence factors such as the Panton-Valentine leukocine (PVL), which are thought to contribute to disease pathogenesis, and can be inhibited by clindamycin and linezolid (33).
Therapeutic principles in current CAP guidelines
The major differences between US and European CAP guidelines center upon recommendations to use penicillins and to avoid fluoroquinolones in the United Kingdom and parts of Europe, compared to the recommendation for routine atypical pathogen coverage in North America, and the view that quinolones can be freely used. The 2007 IDSA/ATS guidelines for CAP classify patients into three groups: outpatients, inpatients not admitted to the ICU, and patients admitted to the ICU (Table 2). Guidelines give direction on these site of care determinations, discussing the use of validated prognostic scores such as CURB-65, the pneumonia severity index, or in the case of ICU admission, meeting one of two major criteria of either need for mechanical ventilation or the presence of septic shock. In the 2007 IDSA/ATS guidelines, ICU admission was also recommended for patients meeting at least three minor criteria, which include: PaO2:FiO2 ratio <250, respiratory rate >30 breaths/minute, confusion, multilobar infiltrates, systolic blood pressure <90 mmHg despite fluid resuscitation, blood urea nitrogen >20 mg/dL, leukopenia (<4,000 cells/mm3), thrombocytopenia (<100,000 cells/mm3), and hypothermia (<36 °C) (17). Other findings indicating severe pneumonia and possibly the need for ICU level of care include hyponatremia (<130 mEq/L) and arterial pH <7.3 (34,35). The recommended therapy for each of these groups differs, because of data showing that the likely pathogens vary in each of these settings, although for all patients, pneumococcus is the number one pathogen.
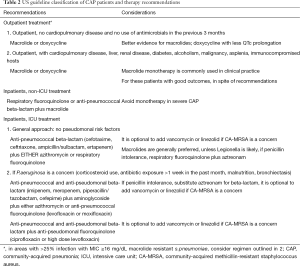
Full table
Empiric CAP recommendations in the US guidelines focus on the need to give the first dose of antibiotics rapidly (between 4 and 6 hours of presentation) and in the emergency department prior to triage. In addition, specific therapy principles include (Table 3): all patients should be treated for atypical pathogens and pneumococcus as well as other pathogens, depending on risk-factors; use of an advanced macrolide as monotherapy only in selected patients; avoiding the repeated use of same agent that the patient was treated with in the past 3 months; use of anti-pseudomonal therapy only in patients with risk factors (corticosteroid use, broad spectrum antibiotic exposure for more than 1 week in the past month, malnutrition, or structural lung disease such as bronchiectasis); cautious use of empiric MRSA therapy, even in critically ill patients; not using intravenous cefuroxime due to concerns of failure in the setting of in vitro pneumococcal resistance (which is not a concern in other guidelines); and lastly that no ICU admitted patient with CAP receive monotherapy.

Full table
These principles translate into the recommendations in Table 2. Outpatients with CAP patients should receive advanced macrolide monotherapy or doxycycline in those with no recent antibiotic use and the absence of cardiopulmonary disease. For outpatients with comorbid illness, recent antibiotic therapy in the past 3 months, or risk factors for DRSP (listed above), therapy should be with an oral β-lactam plus a macrolide or doxycycline, or alternatively, quinolone monotherapy. Although this recommendation is not always followed, and many such outpatients receive macrolide monotherapy in the US, outcomes are generally good. For non-ICU inpatients, combination therapy with a selected intravenous β-lactam (cefotaxime, ceftriaxone, ampicillin/sulbactam, or ertapenem) plus a macrolide or doxycycline, or, an anti-pneumococcal quinolone (levofloxacin 750 mg or moxifloxacin 400 mg) are typically chosen given concerns about DRSP and enteric gram-negative pathogens, especially in those with co-existent cardiopulmonary illness (17). For patients admitted to the ICU, all patients should be treated with combination therapy, and therapy should always be directed against DRSP and atypical pathogens. Patients with severe CAP should be evaluated for pseudomonal risk factors, and those without risks, should receive a non-pseudomonal beta-lactam plus either a macrolide or a quinolone. Those at risk for pseudomonas should be treated with either a two-drug regimen, using an anti-pseudomonal β-lactam (piperacillin/tazobactam, cefepimine, imipenem, meropenem) plus ciprofloxacin or high-dose levofloxacin, or, alternatively a three-drug regimen with an anti-pseudomonal β-lactam plus an aminoglycoside and either an anti-pneumococcal quinolone or a macrolide. In patients with severe CAP and penicillin intolerance, aztreonam plus an anti-pseudomonal quinolone such as levofloxacin, is recommended, although there is scant treatment evidence in this situation. Therapy directed against MRSA should not be used routinely in all patients with severe CAP, but empiric therapy for MRSA should be confined to patients presenting after a viral or influenza infection, with features of necrotizing pneumonia, and should be treated with either vancomycin plus clindamycin, or linezolid alone.
European and British guidelines recommend oral penicillins and tetracyclines for outpatients and discourage the routine use of quinolones and place less emphasis on the need for macrolides, as previously mentioned (16,19,20,36). The latest European guidelines consider as optional the addition of a macrolide to a β-lactam, allowing for the use of penicillin G, for inpatients treated outside of the ICU, and consider quinolones an acceptable option in these patients, favoring moxifloxacin over levofloxacin due to improved anti-pneumococcal activity and high oral bioavailability (16). Although European guidelines support combination therapy for severe pneumonia, they allow for the use of quinolone monotherapy for severe CAP without septic shock. This is related in part to limited data from Europe that illustrates quinolone monotherapy with levofloxacin may be as effective as combination therapy in cases of severe pneumonia, in the absence of pneumonia accompanied by septic shock, or the need for mechanical ventilation (37). The limitations of quinolone monotherapy in severe CAP were echoed in one US study that showed quinolone monotherapy was associated with a two-fold increase in mortality when compared to β-lactam/macrolide therapy in patients with PSI class V pneumonia (38). Of note, MRSA is not common in Europe and therefore recommendations to empirically cover this entity are not included in guidelines from those countries.
Therapy of healthcare-associated pneumonia (HCAP)
A detailed discussion of this topic is beyond the score of this review, however new guidelines will consider HCAP to be a form of community acquired and not nosocomial pneumonia. In prior US guidelines, HCAP was recommended to be treated with multiple, broad-spectrum antibiotics, and this led to overtreatment in some patients. More recently, an algorithm has been developed that leads to only half of HCAP patients being treated as nosocomial pneumonia and the other half are treated as CAP, with over 90% receiving appropriate empiric therapy (39). In this algorithm, patients are first identified as individuals with HCAP risk factors: residence in a nursing home, hospitalization for >2 days in the past 3 months, hospitalization in the last 30 days, home wound care, intravenous antibiotics or chemotherapy in the past 30 days, or exposure to a family member with multidrug-resistant (MDR) pathogens. Patients with HCAP are then divided into 4 groups on the basis of whether they have severe illness, and then risk factors for drug resistant pathogens. These risks include: antibiotic use in the past 6 months, poor functional status, hospitalization in the past 90 days, and immune suppression. Those HCAP patients, with non-severe illness and up to 1 drug resistance risk factor, and those with severe illness and no additional risk factor, were treated with a CAP regimen, while the others were treated with a nosocomial pneumonia regimen. Interestingly, in another study, the benefit of macrolides for mortality in CAP was not seen in HCAP patients (40).
Issues to be addressed in future CAP guidelines
Future CAP guidelines will need to incorporate important recent observations related to the use of biomarkers to inform treatment initiation and length of therapy, considerations regarding the use of adjunctive anti-inflammatory agents (corticosteroids and immunoglobulins), and may incorporate antibiotic therapies that are currently in development (Table 4). In addition, future guidelines need to provide recommendations for when to do cardiac monitoring, now that we understand the high frequency of acute cardiac events complicating CAP; how to prevent cardiac complications of CAP; how to use new treatment modalities such as high flow oxygen to avoid intubation; and how to best utilize current vaccines against pneumococcus and influenza.

Full table
Important data about biomarkers such as procalcitonin, have shown that it may be useful to guide both when to use antibiotics (and when not to, because of findings suggesting viral infection), and for guiding duration of therapy. Currently, CAP therapy is recommended for a minimum of 5 days, provided that the patients is afebrile for 48 hours, has ≤1 clinical instability factor, has received accurate empiric therapy and has no extra-pulmonary site of infection. In this setting, 5 days of therapy is safe and effective, and it is unlikely that biomarkers would impact therapy for these patients (41). However, biomarkers might affect duration of therapy in more severely ill patients, and a randomized controlled open label study by de Jong et al. (42), in the Netherlands, investigated if down trending procalcitonin levels could be used as a guide to discontinue antibiotics in patients—65% with presumed pneumonia—admitted to the ICU. In that study, procalcitonin guidance led to less antibiotic use, a shorter duration of therapy, and a lower 28-day mortality. However, it should be noted that regardless of the procalcitonin level, antibiotic therapy was not stopped early in nearly half of the patients, some of who were considered clinically unstable.
Conclusions
CAP guidelines play an important role in the treatment and management of pneumonia, the world’s leading cause of death from infectious disease. Treatment principles advocated in guidelines is not only associated with reduced mortality and cost of care, but also assist clinicians in choosing evidence-based therapies across the spectrum of pneumonia severity. New guidelines need to be developed in order to incorporate recent observations and advances, including the use of biomarkers and the selective use of corticosteroids, which have been shown to improve outcomes in the treatment of pneumonia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Niederman MS, Luna CM. Community-acquired pneumonia guidelines: a global perspective. Semin Respir Crit Care Med 2012;33:298-310. [Crossref] [PubMed]
- Örtqvist A. Treatment of community-acquired lower respiratory tract infections in adults. Eur Respir J Suppl 2002;36:40s-53s. [Crossref] [PubMed]
- Arnold FW, LaJoie AS, Brock GN, et al. Community-Acquired Pneumonia Organization (CAPO) Investigators. Improving out-comes in elderly patients with community-acquired pneumonia by adhering to national guidelines: Community-Acquired Pneumonia Organization International cohort study results. Arch Intern Med 2009;169:1515-24. [Crossref] [PubMed]
- Blasi F, Iori I, Bulfoni A, et al. Can CAP guideline adherence improve patient outcome in internal medicine departments? Eur Respir J 2008;32:902-10. [Crossref] [PubMed]
- Capelastegui A, España PP, Quintana JM, et al. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis 2004;39:955-63. [Crossref] [PubMed]
- Shorr AF, Bodi M, Rodriguez A, et al. CAPUCI Study Investigators. Impact of antibiotic guideline compliance on duration of mechanical ventilation in critically ill patients with community-acquired pneumonia. Chest 2006;130:93-100. [Crossref] [PubMed]
- Bodí M, Rodríguez A, Solé-Violán J, et al. Community-Acquired Pneumonia Intensive Care Units (CAPUCI) Study Investigators. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis 2005;41:1709-16. [Crossref] [PubMed]
- Menéndez R, Ferrando D, Vallés JM, et al. Influence of deviation from guidelines on the outcome of community-acquired pneumonia. Chest 2002;122:612-7. [Crossref] [PubMed]
- McCabe C, Kirchner C, Zhang H, et al. Guideline- concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Ann Intern Med 2009;169:1525-31. [Crossref] [PubMed]
- Simpson SH, Marrie TJ, Majumdar SR. Do guidelines guide pneumonia practice? A systematic review of interventions and barriers to best practice in the management of community-acquired pneumonia. Respir Care Clin N Am 2005;11:1-13. [Crossref] [PubMed]
- Vergis EN, Yu VL. New directions for future studies of community- acquired pneumonia: optimizing impact on patient care. Eur J Clin Microbiol Infect Dis 1999;18:847-51. [Crossref] [PubMed]
- Arnold FW, Summersgill JT, Lajoie AS, et al. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med 2007;175:1086-93. [Crossref] [PubMed]
- World Health Organization, Department of Health Statistics and Informatics. Estimates of Deaths by Cause for the Year 2008. Available online: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf
- Working Group of the South African Thoracic Society. Management of community-acquired pneumonia in adults. S Afr Med J 2007;97:1296-306. [PubMed]
- Gleason PP, Meehan TP, Fine JM, et al. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med 1999;159:2562-72. [Crossref] [PubMed]
- Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005;26:1138-80. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guide-lines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27-72. [Crossref] [PubMed]
- Niederman MS, Brito V. Pneumonia in the older patient. Clin Chest Med 2007;28:751-71. [Crossref] [PubMed]
- Woodhead M, Blasi F, Ewig S, et al. Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiolo- gy and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Micro- biol Infect 2011;17:E1-59. [Crossref]
- British Thoracic Society Standards of Care Committee. BTS Guidelines for the Management of Community Acquired Pneumonia in Adults. Thorax 2001;56:IV1-64. [Crossref] [PubMed]
- Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 2004;170:440-4. [Crossref] [PubMed]
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001;161:1837-42. [Crossref] [PubMed]
- Martin-Loeches I, Lisboa T, Rodriguez A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med 2010;36:612-20. [Crossref] [PubMed]
- García Vázquez E, Mensa J, Martinez JA, et al. Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur J Clin Microbiol Infect Dis 2005;24:190-5. [Crossref] [PubMed]
- Asadi L, Sligl WI, Eurich DT, et al. Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis 2012;55:371-80. [Crossref] [PubMed]
- Sligl WI, Asadi L, Eurich DT, et al. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med 2014;42:420-32. [Crossref] [PubMed]
- Feikin DR, Schuchat A, Kolczak M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health 2000;90:223-9. [Crossref] [PubMed]
- Vanderkooi OG, Low DE, Green K, et al. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis 2005;40:1288-97. [Crossref] [PubMed]
- Tseng YT, Chuang YC, Shu CC, et al. Empirical use of fluoroquinolones improves survival of critically ill patients with tuberculosis mimicking severe pneumonia. Crit Care 2012;16:R207. [Crossref] [PubMed]
- Weiss K, Tillotson GS. The controversy of combination vs mono- therapy in the treatment of hospitalized community-acquired pneumonia. Chest 2005;128:940-6. [Crossref] [PubMed]
- Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis 2000;31:347-82. [Crossref] [PubMed]
- Lobo LJ, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest 2010;138:130-6. [Crossref] [PubMed]
- Diep BA, Afasizheva A, Le HN, et al. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and survival outcomes in a rabbit model of MRSA necrotizing pneumonia. J Infect Dis 2013;208:75-82. [Crossref] [PubMed]
- Shindo Y, Sato S, Maruyama E, et al. Comparison of severity scoring systems A-DROP and CURB-65 for community-acquired pneumonia. Respirology 2008;13:731-5. [Crossref] [PubMed]
- Nair V, Niederman MS, Fishbane S. Hyponatremia in community acquired pneumonia. Am J Nephrol 2007;27:184-90. [Crossref] [PubMed]
- File TM Jr, Garau J, Blasi F, et al. Guidelines for empiric antimicrobial prescribing in community-acquired pneumonia. Chest 2004;125:1888-901. [Crossref] [PubMed]
- Leroy O, Saux P, Bédos JP, et al. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest 2005;128:172-83. [Crossref] [PubMed]
- Lodise TP, Kwa A, Cosler L, et al. Comparison of beta- lactam and macrolide combination therapy versus fluoroquino-lone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother 2007;51:3977-82. [Crossref] [PubMed]
- Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis 2013;57:1373-83. [Crossref] [PubMed]
- McEvoy C, Micek ST, Reichley RM, et al. Macrolides are associated with a better survival rate in patients hospitalized with community-acquired but not healthcare-associated pneumonia. Surgical infections 2014;15:283-9. [Crossref] [PubMed]
- Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016;176:1257-65. [Crossref] [PubMed]
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016;16:819-27. [Crossref] [PubMed]
Cite this article as: Bender MT, Niederman MS. Treatment guidelines for community-acquired pneumonia. Ann Res Hosp 2018;2:6.


