
Lipid peroxidation products and their role in neurodegenerative diseases
Introduction
Oxidative stress is a biological circumstance driven by the imbalance between prooxidant and antioxidant equilibrium (1). This disequilibrium between free radical production and cellular antioxidants occurs in numerous conditions involving carcinogenesis, inflammation, and neurodegeneration. Defective homeostasis of redox active metals, including iron, leads to generation of neurotoxic reactive species. Redox cyclic between ferrous and ferric iron is utilized in the biology of various electron transfer reactions essential to a number of cellular processes. These include neurotransmitter synthesis, myelination of neurons and mitochondrial function. When this chemistry is altered deleterious reactions occur with oxygen inducing oxidative stress (2,3). Lipid peroxidation (LPO) is a fundamental constituent of oxidative stress and free radical production (4). In particular, reactive oxygen species (ROS) are able to attack polyunsaturated fatty acids (PUFAs) of cellular membranes, leading to functional and/or structural impairment of the membranes, eventually generating a group of α, β-unsaturated highly reactive aldehydes, among which 4-hydroxy-2-noneal (HNE), malondialdehyde (MDA) and acrolein being the most reactive (5). Consequently, these strong reactive aldehydes are greatly diffusive and capable of attacking or forming covalent linkages with farther cellular constituents (5). Ensuing this process, LPO continues self-propagation and initiation of chain reactions that are terminated either with complete substrate utilization or via interaction with antioxidants such as vitamin E (3,5). Among others, isoprostanes (IsoPs) and neuroprostanes (neuroPs) are additional LPO products of arachidonic acid and docosahexaenoic acid (DHA), respectively (4,5). These cyclized fatty acids proliferate further, attacking cellular membrane components, mainly lipids and proteins, and propagating other LPO products (4,5).
Quantification of ROS, either in vivo or in vitro, is challenging due to their short half-lives (6). Thus, oxidation products are, instead, quantified in biological samples, in which the magnitude of oxidative stress is quantifiable by measuring the more stable oxidation products (5,7). In respect of their oxidative-induced damage properties, these compounds are considered as disease mediators in the pathophysiology of many neurodegenerative diseases (NDs), including Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) (8). The role of LPO in the pathogenesis of these neurodegenerative disorders has been substantially founded, since markers of oxidative impairment have been identified in elevated levels in brain tissues and bodily fluids, suggesting their dual role as both disease mediators and potential biomarkers (7). The aim of the present review is to describe LPO process and its products, and to characterize their role as potential biomarkers in NDs.
LPO
Chemistry of LPO
LPO indicates the oxidative deterioration of lipids occurring via a five-step sequential procedure and can be generally described as an event in which oxidants, either radical or non-radical species, attack lipids containing C-C double bonds (9,10). Contrary to enzymatic lipid metabolism, lipoid peroxidation is a non-enzymatic process that proceeds in an uncontrolled manner via three distinctive phases, i.e., the initiation, propagation and termination (11) (Figure 1).
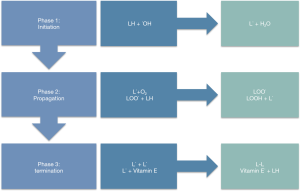
Specifically, in the initiation phase, free radicals (involving hydroxyl, alkoxyl, or peroxyl radicals) attack a methylene group in the PUFAs, eventuating in an allylic H atom dissociation along with the rearrangement of the double bonds in the diene conjugated pattern, while generating a C-concentrated lipid radical (L˙) (5,9,10). In the second phase, the alkyl radicals are reacting with paramagnetic molecular O2 forming the peroxyl radical (LOO˙), while in the third phase propagation commences (9,10) (Figure 1). In this phase, the peroxyl radical abstracts a further H allylic atom, through which a self-sustained chain reaction is triggered, leading to the amplification of the initial oxidative event (9,11). Through this process the plurality of membrane lipids is modified to a plethora of cyclic peroxides and hydroperoxides (LOOH˙) (5,10). Accordingly, the hydroperoxides can be further degraded to aldehydes, giving rise to MDA, HNE and acrolein. These aldehydes induce irreversible alteration of phospholipids, proteins, and DNA, leading to functional impairment as cyclic peroxides ascent to neuroprostanes and isoprostanes (9). Following that step, the fourth step involves the termination of lipoid peroxidation; in which horizontal interplay among dissimilar types of radicals obstruct the chain reaction cascade (8,9). Eventually, in the fifth step, termination occurs via interaction between radicals and antioxidants, resulting in the production of non-reactive radicals, or non-radical products (5,9,10). In particular, a lipid radical (L˙) reacts with lipid peroxide (LOO˙), or two peroxide molecules are combined, resulting in non-reactive species (LOOL) or hydroxylated derivatives (LOH˙); both being chemically stable (5,7,10). In the termination process, antioxidants can be of either exogenous or endogenous nature (9,12,13). These include vitamin E and C, which act by abating the generation of LPO at the initial stages of the free radical attack, operating as chain breaking antioxidants (9,14) (Figure 1).
Means of action of LPO
Membrane LPO influences numerous functions leading to elevated membrane rigidity, diminished action of membrane-confined enzymes, damage of membrane receptors and modified permeability of the cell membrane (7,15). Similar to phospholipid impairment, radicals can directly attack membrane proteins and mediate lipid-protein as well as protein-protein interconnection, which additionally affect membrane integrity (7,12). Out of all these events, LPO products induce such a loss of membrane integrity that eventually leads to loss of cellular function and severe cytotoxicity, and could result in uncontrolled cellular growth or even apoptosis (2,3,13,15). Reasonably, the perturbation of the aforementioned functions occurred by PUFAs, together with the consequent metabolites and protein modifications disturb the neuronal homeostasis, thus, leading to brain dysfunction (7,13).
LPO products
Malondialdehyde
Malondialdehyde (MDA) is an extremely reactive and toxic aldehyde, generated by PUFAs peroxidation and deriving from decomposition of certain LPO products (5,16). Additionally, MDA can be a consequence of prostaglandin breakdown by the cyclooxygenase activity, or via various non-lipid precursors, involving amino acids and carbohydrates that are able to produce MDA (5,15). MDA is capable of interacting with nucleic acid bases, forming dissimilar adducts, and can also react with proteins in a synergistic and covalent manner, eventuating in stable protein adducts that further result in the induction of strong immune responses and exhibit pro-fibrogenic and pro-inflammatory properties (14,16). Furthermore, assemblage of MDA is able to modify membrane permeability and deteriorate the fluidity of membrane lipid bilayer (5,16). MDA is one of the most mutagenic LPO products, being capable of reacting with deoxyguanosine and deoxyadenosine in DNA, thus generating mutagenic DNA adducts (5).
4-Hydroxy-2-nonenal (HNE)
HNE is a α, β-unsaturated alkenal formed as a consequence of peroxidation of ω-6PUFAs, essentially the linoleic and arachidonic acid; since they are the most abundant fatty acids (12,17). HNE displays three functional groups, the aldehyde, C=C and hydroxyl group that, in sequence or alone, engage in chemical reactions with various molecules (16). More specifically, HNE is able to bind to cysteine, histidine and lysine proteinaceous residues via Michael addition by either the amino (-NH2) or thiol (-SH) groups (5,12). This aldehyde is highly electrophilic and capable of reacting with DNA, proteins and glutathione, exhibiting a hydrophobic nature (9,16). Protein residues, bound to HNE, can damage normal protein function as well as structure, and HNE exhibits reactivity with vital cellular components including nucleic acids, lipids, signaling molecules and vitamins (5,15,16). Due to its electrophilic properties, HNE possesses the potential of involvement in modulating enzyme action, gene expression and signal transduction, while its adducts contribute to enzyme damage that is elevated during aging and in specific pathological conditions (4,12,14). Specifically, HNE interacts and interferes with normal cellular activities involving glucose uptake at synapses further contributing to synaptic deterioration, while it also induces loss of organelle function, e.g., microtubule dysfunction (14). HNE is capable of accumulating in extremely low concentrations, such as 10µM, in response to oxidative stress and evokes cytotoxicity and selective suppression of basal and inducible NF-κB (9). Elevated levels of HNE cause disruption of glutamate transport, Ca2+ homeostasis, microtubule function, membrane impairment and cellular death via the activation of caspase pathways (17). Antithetically, even diminished grades of HNE are able to increase and modify susceptibility of proteolysis and removal via the proteasomal system (9,17).
Acrolein
Acrolein is the most reactive product of LPO and exhibits a structure with three C atoms and a double bond (14,16). In addition to PUFAs, acrolein is generated by threonine metabolism of activated phagocytes and cyclophosphamide bio-activation (5,16). More specifically, acrolein is an electrophilic compound that binds or interacts extremely rapidly with fundamental cellular enzymes and nucleophiles, leading to their deactivation or deficiency (5,16). Moreover, acrolein reacts with nucleophilic sites in DNA and proteins, leading to the formation of DNA and protein adducts and, thus, exerting cytotoxicity primarily related to its ability to diminish glutathione, (5). Moreover, targets of acrolein in proteins involve the side chains of histidyl, lysyl, and cysteinyl residues as well as free N-terminal amino groups (5,14,15). Cysteinyl residues are encountered in the active sites of proteins and are implicated in the catalytic action of enzymes; therefore, the formation of acrolein-cysteine adducts present a wide spectrum of functional implications (16). This interaction causes alteration of cysteinyl residues that further cause inactivation of enzymes (14-16). Broadly, acrolein is capable of inducing cellular degeneration and death, and particularly deterioration of hippocampal neurons (5).
Isoprostanes
The isoprostanes (IsoPs) also indicated as F2-isoprostanes, are prostaglandin-resembling compounds generated via free radical-compelled oxidation of arachidonic acid and further discharged from membrane phospholipids via phospholipases (16). IsoPs exhibit a stable nature when detected in biological samples, while their aggregation can impair the integrity and fluidity of cellular membranes (14-16). Similar to prostaglandins, they possess a fundamental action in cellular proliferation, platelet function, nociception, and smooth muscle contraction (17).
Neuroprostanes
Neuroprostanes (NeuroPs), or also annotated as F4-isoprostanes, are produced by free radicals catalyzing oxidation of docosahexaenoic acid (DHA) (5). DHA is a vital compound of the nervous tissue, greatly enriched in neurons and profoundly vulnerable to oxidation (4). In a biological aspect, neuroPs depict anti-inflammatory properties while inhibiting proteasome activity (17). Additionally, neuroPs are abundantly concentrated in the neuronal membranes (16).
NDs and LPO products
The central nervous system (CNS) is one the major targets of the LPO, actually being particularly vulnerable, since the composition of neuronal tissue is susceptible to chain reactions moderated by free radicals, which eventually result in LPO products (7,18). The CNS consists of elevated grades of PUFAs and redox transition metal ions besides its excessive consumption of oxygen (9). The redox transition metals are fundamental in the initiation and propagation processes that commence by the abstraction of an electron from the conjugate double bond fatty acid acyl chain system (9,12). Additionally, the levels of enzymatic antioxidants are comparatively diminished and probably contribute to the accumulative process of oxidative impairment (7,9). The role of LPO in the pathogenesis of NDs is of paramount importance and deeply associated especially with the neurodegenerative disorders described in the following sections (17).
AD and LPO products
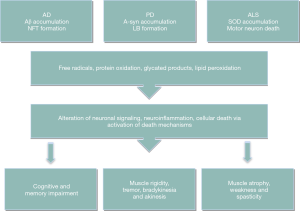
AD is typified by amyloid β peptide (Aβ) deposition and microtubule-related tau protein in the CNS (19,20). The hallmarks of AD involve plaque formation via Aβ overproduction and, ultimately, its accumulation via the cleavage of amyloid precursor protein (APP) into the two products Αβ40 and Αβ42. This accumulation results in the production of the senile plaques. The second hallmark includes the composition of neurofibrillary tangles (NFTs) via the hyperphosphorylation of tau protein (21,22). AD may be inherited or sporadic, involving genetic mutations in APP, presenilin 1, presenilin 2 and α-2-macroglobulin genes (17). In the occurrence of AD pathology, aberrant tau phosphorylation is fundamental, while Aβ possesses the ability to interact with transition metals, which, in turn, develop redox active ions that precipitate in LPO (17,20). In general, AD affects regions of the CNS that are responsible for memory and cognitive functions, which progressively, apart from memory impairment, diminish patient’s ability of learning, reasoning, and communicating (17,21).
Interestingly, it has been reported that protein grades of antioxidant enzymes, as well as their activity, were altered in particular CNS domains, consonantly with the progressive oxidative stress (Figure 2). LPO process occurs in every stage of the AD, consisting of mild cognitive impairment (MCI), early stage AD and late stage AD (17,20). More specifically, in MCI early amnestic event, high grades of HNE and acrolein together with high levels of F2-IsoPs and F4-NeuroPs have been reported in specific domains of the CNS, involving parietal, frontal and occipital lobes and suggesting their contribution to memory deficiency (17).
HNE, as protein bound, induces conformational and, ultimately, functional alterations to proteins such as, among others, α-enolase and ATP-synthase (12,17). A-enolase catalyzes the conversion of 2-phosphoenolpyruvate to phosphoenolpyruvate in glycolysis. More specifically, α-enolase exhibits elevated oxidation in all stages of AD, along with diminished enzyme action. HNE-induced modification of α-enolase leads to alteration in energy metabolism, widely seen in NDs (12,17,18,20). Thus, protein conversion of α-enolase probably disrupts neuronal energy metabolism and ion homeostasis through which membrane ion motive ATPases, glucose and glutamate transporters function is impaired. This event causes loss of signal transduction and membrane asymmetry (7,18), thereby leading to neuronal vulnerability to excitotoxicity and apoptosis (17) (Figure 2). As for the ATP-synthase, a mitochondrial coordinating factor that undergoes structural alterations in order to produce ATP, its modification by HNE has been identified in each AD stage (12,17,23). Oxidation of ATP-synthase leads to inactivation of that complex mitochondrial function (17). ATP-synthase functional failure contributes to diminished action of the entire electron transport chain (ETC) and disruption of ATP production. This event potentially leads to electron leakage from the carrier molecules, thus producing ROS, as altered expression of mitochondrial proteins, decreased activity of numerous ETC complexes and functional deficiencies are well-established in AD (12,17,20). On the other hand, MDA exhibits similar protein repertoire with HNE, including the formation of α-/γ-enolase and ATP-synthase adducts, among of other proteins (16).
Additionally, acrolein has been identified in the temporal cortex and hippocampus in which the oxidative stress levels are high (16). Acrolein exhibits extreme neurotoxic affects and induces its adverse effects by forming adducts and protein cross-linking (16,18). Moreover, there is evidence that acrolein acts primarily in the nerve terminals, causing synaptic impairment prior to neurotoxicity and, overall, leading to diminished presynaptic neurotransmitter discharge (16). More specifically, acrolein-induced presynaptic membrane neurotransmitter uptake and vesicular storage inhibition is linked with adducts formation with dopamine transporter and v-ATPase (18). In in vitro studies it was depicted that these events lead to reduced glutamate uptake, impaired synaptosomal membrane protein conformation and phospholipid asymmetry (4,21). Lastly, reduced glucose transporter 3 (GLUT3)-mediated glucose transport, decreased respiration, oxidative stress in synaptosomal mitochondria, obstructed Na+ and Ca2+ ion pumps, and disrupted ion regulation were reported in nerve culture cells (16,24,25).
The defective homeostasis of iron probably contributes to AD neuropathology as well. To specify, high levels of iron have been identified in co-localization with insoluble amyloid plaques and NFTs. Increased iron concentration has been implicated in the misfolding of the processes associated with amyloid beta production from APP, hyperphosphorylated Tau and contributing to neuronal oxidative stress (26-28).
PD and LPO products
PD is attributed to protein aggregates of α-synuclein (α-syn), which primarily exhibits function in mitochondrial processes and for the formation of synaptic vesicles (17). PD is both sporadic and familial, as genetic mutations in parkin and ubiquitin carboxy-terminal hydroxylase 1 (UCHL1) genes are probably associated with PD occurrence (17). Misfolded α-syn is soluble and a probable mediator of neurotoxicity in dopaminergic neurons, resulting in apoptosis mediated by ROS (15). In addition, Lewy bodies (LBs) inclusions are also presented in cerebrospinal cord, enteric nervous system and autonomic ganglia (29,30) and encompass a dense center of granular and filamentous material, which is encircled by branching fibrils composed mainly of α-syn (29,30). PD is characterized by specific degeneration of dopaminergic neurons of the substantia nigra (21). Ultimately, PD pathology leads to diminishment of motor function, resulting in resting tremors, muscle rigidity, bradykinesia and eventually akinesia (17) (Figure 2).
Regarding the role of LPO in PD, acrolein has been associated with α-syn modification in dopaminergic neurons, ultimately causing mitochondrial dysfunction (17). LPO grades are increased in PD by elevated levels of HNE, MDA acrolein, isoPs and neuroPs (17,30). Moreover, HNE and MDA have been reported in LBs, in the brain stem and neocortical neurons (15). HNE and MDA adducts are prone to accumulate in neocortical and brain stem neuronal cells (16). Under conditions characterized by high LPO, dopamine is oxidatively converted to o-quinone, which in turn commences a series of spontaneous reactions. In this case, intramolecular cyclization and molecular interaction with specific targets leads to cytotoxic responses and changed cell functioning (16). Accordingly, HNE has been found to modify transport and probably loss of dopamine, which is fundamental in the pathogenesis of the disease (17) (Figure 2). Thus, HNE elevation, protein accumulation and dopamine loss eventually affect learning and physical capabilities of PD patients (17). Moreover, HNE advances the generation of oligomers with seeding capabilities, providing a cell-to-cell transferring ability (31). HNE-altered oligomers are possibly toxic and contribute to the neuronal loss induced by oxidative impairment (16,32). Since the fatty acid-fastening protein transfers fatty acids and lipophilic molecules across biological membranes, an elevation in the overall grades of these proteins is capable of inducing increased oxidative stress due to high substrate concentration (16).
In PD, the most affected brain domain is that of the substantia nigra, in which α-enolase and β-actin were identified to be oxidatively modified in animal models (17). Neurofilament light (NF-L) has also been detected to be substantially decreased in PD and in the substantia nigra as well (16). Neurofilaments act as components of the axonal cytoskeleton and NF-L is the most abundant one. It is capable of self-assembling as it is found in the LBs (17,33). Acrolein presence induces protein aggregation of NF-L, which is identified as a phosphatase-1-binding protein (17). These assemblages exhibit cross-linking abilities and generate oxidative stress via increased grades of protein carbonyls (17). Furthermore, HNE has been reported in NF-L, suggesting a correlation between LPO and neuronal loss (32,34) (Figure 2).
Another protein of actin-fastening origin has been reported in cell lines and it acts to regulate the development of actin microfilament, which is vital in cellular kinesis and morphology (17). Upon oxidation, this protein is decreased in PD, leading to diminished restoration of dynamic neuronal development, which ultimately results in muscular impairments widely observed in PD (17,30). Additionally, DHA was identified to induce stimulation of α-syn oligomerization, thus further promoting the formation of α-syn assemblages (21) and suggesting that DHA has a potential role in α-syn aggregate formation, as its levels are increased in PD brains (21,35,36).
Iron accumulation can lead to induction of ROS generation mediating dopaminergic neuronal death by causing oxidation protein, lipids, and other macromolecules in the cells. Dopamine is an unstable molecule that may auto-oxidize to form quionones and H2O2. This can react with iron or oxygen to form the reactive OH˙. Increased levels of iron are observed in the substantia nigra of PD patients and has been observed in primary dopaminergic neurons in vitro that iron loading increased a-syn and ORS levels (37-41).
ALS and LPO products
ALS is a degenerative disease that exhibits a multifactorial nature and is both of sporadic and hereditary origin. Interactions between genetic and environmental factors, or solely each of these factors, result in motor neural degeneration (19,21). Clinical characteristics of ALS pathology involve muscle atrophy, weakness and spasticity (17,21) (Figure 2). A characteristic deposition of superoxide dismutase (SOD), either of copper or zinc protein, occurs in a misfolded manner in the neural tissue (18,19). Inherited ALS presents mutation in the SOD antioxidant enzyme, which results in cellular toxicity, as the capability of reducing the toxic free radicals is reduced (17). In ALS, neuro-inflammatory alterations are found including elevated grades of pro-inflammatory molecules, microglial activation and astrogliosis (8,17), suggesting that inflammation may probably promote motor neural death (19). LPO is enhanced in ALS via the mutation of the antioxidant enzyme SOD and the high increase of LPO products, which lead to neuronal loss (17) (Figure 2). More specifically, three HNE-modified proteins, including dihydropyrimidinase-related protein 2 (DRP-2), heat shock protein 70 and α-enolase, are presented in this disorder (17,18). DRP-2 is involved in the axonal development and in guiding via transmission and adjusting extracellular signals (19). DRP-2 is able to fasten to tubulin heterodimers and bundle microtubules, thus promoting microtubule dynamics and assemblage (19). Ergo, motor neurons are affected as they are composed by actin microfilaments and microtubules (19). Modification of DRP-2 by HNE further leads to a poor muscle control (8,19,30). Additionally, HNE-modified proteins include collapsin response mediator protein-2 (CRMP-2), which is involved in the axonal development, in leading via transmission and modulation of extracellular signals, and in exhibiting repairing capabilities. A loss of repairing ability is probably correlated with oxidation of CRMP-2, which leads to the loss of that particular function (9,30). Finally, LPO appears to play a fundamental role in the pathophysiology of the motor neuron disorders (Figure 2), and particularly in the context of SOD mutations, as HSP70 that binds to HNE leads to inactivation of its protective properties against mutated SOD misfolding (9).
The perspectives of using LPO products as biomarkers
A wide number of LPO products have been utilized for assessing the status of LPO in vivo, while DNA bases and proteins modified by LPO products have also been used as biomarkers (42,43). The most frequently exploited LPO products are that of lysine residues and unsaturated aldehydes, including HNE and acrolein (42). Many studies have been investigating the interrelation of LPO products and disease state, including the application of possible biomarkers in order to assess prognosis and establish early detection (34,42).
Among the aforementioned potential biomarkers, IsoPs provide the most reliable and robust outcomes (7,43). Moreover, the most accurate process of assessing oxidant stress in vivo is the quantification of plasma and urinary IsoPs (7). IsoPs have been identified in increased grades in human bodily fluids and tissues in a vast number of diseases including the NDs (43). Yet, determining the IsoPs normal range in healthy individuals is vital, as it permits the actuation of the magnitude to which any means of therapeutic intervention may affect oxidative stress grades (7,18). In addition, IsoPs are composed in situ on phospholipids, at locations of free radical production, while upon discharge from the cellular membranes via phospholipases IsoPs circulate in the plasma (44). In general, IsoP have been detected and measured in a plethora of biological fluids including urine, exhaled breath condensate, plasma cerebrospinal fluid, and bile (44,45). However, IsoPs are encountered as marginal oxidation products of the arachidonic acid while there are many other isomers produced through diverse reactions (43,44). In general, IsoPs are not widely abundant in vivo and particularly in the human plasma (44). Hence, the handling, analysis or storage of an artificial oxidation amid sample is a potential concern (43,44).
On the other hand, neuroPs are a fundamental component of the nervous tissue, which are increasingly enriched in the neuronal tissue and extremely susceptible to oxidation (4,23). Quantification of neuroPs provides a sensitive basis of oxidative neuronal damage in parallel to IsoPs quantification, as the grades of neuroPs produced from the DHA surpass that of IsoPs from the arachidonic acid by 3.4 folds (4). Regarding the role of free radicals in CNS and ultimately in NDs pathogenesis, quantification of neuroPs levels is a vital tool in evaluating brain oxidative impairment (4). Similar to isoPs, neuroPs are elevated in the cerebrospinal fluid and brain tissue in ND, such as PD and AD; even though neuroPs are markedly more elevated than isoPs in the affected areas of each disorder (4,43,46,47).
Concluding remarks
Oxidation compounds possess the ability of targeting and attacking lipids, thus commencing the LPO process. This process is a chain reaction that results in the production of numerous breakdown molecules including HNE, MDA, acrolein, isoprostanes and neuroprostanes. Additionally, these LPO products are capable of modifying proteins and creating protein adducts that are highly susceptible to these aldehydes, eventually leading to their modification. In particular, HNE, MDA and acrolein adducts are critical in a number of cellular processes and engage in secondary deleterious conversions, such as cross-linking. Cross-linking is a major factor in the development of pathology due to the promotion of intramolecular or intermolecular DNA and protein cross-linking, which results in intense change in the biochemical properties of various biomolecules (48). Particularly in neurodegenerative disorders, those LPO products have been identified in AD, PD and ALS, exhibiting their toxic properties in these diseases. Yet, the exact mechanism of their toxic action is still elusive. In general, this mechanism is thought to be a cascade of chain reactions commenced by covalent interrelation with nucleophilic compounds. Clinical symptoms have been translated and interconnected with specific modified proteins in the CNS, which exhibit those specific cellular alterations that, in long term, are associated with the pathophysiology NDs. The aforementioned neurodegenerative disorders exhibit similar and overlapping protein dysfunctions, including those of α-enolase and ATP synthase, often sharing similar functions. This is consistent with the notion that modified energy metabolism; mitochondrial impairment and diminished antioxidant defenses are typical features of the neurodegenerative disorders. In conclusion, these data provide a broader spectrum of knowledge regarding the development and utilization of LPO products as potential biomarkers. Such biomarkers would contribute to the earlier diagnosis of the disease and shed more light for a better understanding of the associated, impaired metabolic processes, revealing potential targets to alter disease progression.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Spickett CM, Pitt AR. Protein oxidation: role in signalling and detection by mass spectrometry. Amino Acids 2012;42:5-21. [Crossref] [PubMed]
- Ward RJ, Zucca FA, Duyn JH, et al. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014;13:1045-60. [Crossref] [PubMed]
- Popa-Wagner A, Mitran S, Sivanesan S, et al. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev 2013;2013:963520. [Crossref] [PubMed]
- Signorini C, De Felice C, Durand T, et al. Isoprostanes and 4-hydroxy-2-nonenal: markers or mediators of disease? Focus on Rett syndrome as a model of autism spectrum disorder. Oxid Med Cell Longev 2013;2013:343824. [Crossref] [PubMed]
- Erejuwa OO, Sulaiman SA, Ab Wahab MS. Evidence in support of potential applications of lipid peroxidation products in cancer treatment. Oxid Med Cell Longev 2013;2013:931251. [Crossref] [PubMed]
- Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med 2003;24:293-303. [Crossref] [PubMed]
- Sultana R, Perluigi M, Allan Butterfield D. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 2013;62:157-69. [Crossref] [PubMed]
- Barrera G, Gentile F, Pizzimenti S, et al. Mitochondrial Dysfunction in Cancer and Neurodegenerative Diseases: Spotlight on Fatty Acid Oxidation and Lipoperoxidation Products. Antioxidants (Basel) 2016;5. [Crossref] [PubMed]
- Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies. Antioxid Redox Signal 2012;17:1590-609. [Crossref] [PubMed]
- Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014:360438. [Crossref] [PubMed]
- Bradley-Whitman MA, Lovell MA. Biomarkers of lipid peroxidation in Alzheimer disease (AD): an update. Arch Toxicol 2015;89:1035-44. [Crossref] [PubMed]
- Shoeb M, Ansari NH, Srivastava S, et al. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem 2014;21:230-7. [Crossref] [PubMed]
- Yadav UC, Ramana KV. Regulation of NF-kappaB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid Med Cell Longev 2013;2013:690545. [Crossref] [PubMed]
- Fritz KS, Petersen DR. An overview of the chemistry and biology of reactive aldehydes. Free Radic Biol Med 2013;59:85-91. [Crossref] [PubMed]
- Guéraud F, Atalay M, Bresgen N, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res 2010;44:1098-124. [Crossref] [PubMed]
- Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med 2011;51:1302-19. [Crossref] [PubMed]
- Pizzimenti S, Ciamporcero E, Daga M, et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol 2013;4:242. [Crossref] [PubMed]
- Shichiri M. The role of lipid peroxidation in neurological disorders. J Clin Biochem Nutr 2014;54:151-60. [Crossref] [PubMed]
- Thanan R, Oikawa S, Hiraku Y, et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci 2014;16:193-217. [Crossref] [PubMed]
- Miller E, Morel A, Saso L, et al. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid Med Cell Longev 2014;2014:572491. [Crossref] [PubMed]
- Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim Biophys Acta 2010;1801:924-9. [Crossref] [PubMed]
- Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem 2008;49:241-68. [Crossref] [PubMed]
- Chauhan V, Chauhan A. Oxidative stress in Alzheimer's disease. Pathophysiology 2006;13:195-208. [Crossref] [PubMed]
- Peña-Bautista C, Vigor C, Galano JM. Plasma lipid peroxidation biomarkers for early and non-invasive Alzheimer Disease detection. Free Radic Biol Med 2018;124:388-94. [Crossref] [PubMed]
- Pinz MP, Dos Reis AS, Vogt AG, et al. Current advances of pharmacological properties of 7-chloro-4-(phenylselanyl) quinoline: Prevention of cognitive deficit and anxiety in Alzheimer’s disease model. Biomed Pharmacother 2018;105:1006-14. [Crossref] [PubMed]
- Sun Y, Pham N, Waite D. Elucidation of the interplay between Fe(II), Fe(III), and dopamine with relevance to iron solubilization and reactive oxygen species generation by catecholamines. J Neurochem 2016;137:955-68. [Crossref] [PubMed]
- Asano T, Koikec T, Sakata S, et al. Possible involvement of iron-induced oxidative insults in neurodegeneration. Neurosci Lett 2015;588:29-35. [Crossref] [PubMed]
- Schipper HM. Neurodegeneration with brain iron accumulation — Clinical syndromes and neuroimaging. Biochim Biophys Acta 2012;1822:350-60. [Crossref] [PubMed]
- Goedert M, Spillantini MG, Crowther RA. A Brief History of Tau. Clin Chem 2015;61:1417-8. [Crossref] [PubMed]
- Visanji NP, Brooks PL, Hazrati LN, et al. The prion hypothesis in Parkinson's disease: Braak to the future. Acta Neuropathol Commun 2013;1:2. [Crossref] [PubMed]
- Chen RH, Wislet-Gendebien S, Samuel F, et al. alpha-Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity. J Biol Chem 2013;288:7438-49. [Crossref] [PubMed]
- Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis 2013;3:461-91. [PubMed]
- Nam TG. Lipid peroxidation and its toxicological implications. Toxicol Res 2011;27:1-6. [Crossref] [PubMed]
- Praticò D. The neurobiology of isoprostanes and Alzheimer's disease. Biochim Biophys Acta 2010;1801:930-3. [Crossref] [PubMed]
- Csala M, Kardon T, Legeza B, et al. On the role of 4-hydroxynonenal in health and disease. Biochim Biophys Acta 2015;1852:826-38. [Crossref] [PubMed]
- García-Blanco A, Peña-Bautista C, Oger C. Reliable determination of new lipid peroxidation compounds as potential early Alzheimer Disease biomarkers. Talanta 2018;184:193-201. [Crossref] [PubMed]
- Goedert M, Cheng Y. Parkinson's disease: Crystals of a toxic core. Nature 2015;525:458-9. [Crossref] [PubMed]
- Shamoto-Nagai M, Hisaka S, Naoi M, et al. Modification of α-synuclein by lipid peroxidation products derived from polyunsaturated fatty acids promotes toxic oligomerization: its relevance to Parkinson disease. J Clin Biochem Nutr 2018;62:207-12. [Crossref] [PubMed]
- Sun Y, Pham AN, Waite TD. Mechanism Underlying the Effectiveness of Deferiprone in Alleviating Parkinson's Disease Symptoms. ACS Chem Neurosci 2018;9:1118-27. [Crossref] [PubMed]
- Wan W, Jin L, Wang J, et al. Iron Deposition Leads to Neuronal α-Synuclein Pathology by Inducing Autophagy Dysfunction. Front Neurol 2017;8. [PubMed]
- Weng M, Xie X, Liu C, et al. The Sources of Reactive Oxygen Species and Its Possible Role in the Pathogenesis of Parkinson’s Disease. Parkinsons Dis 2018;2018:9163040. [Crossref] [PubMed]
- Farooqui T, Farooqui AA. Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson's disease. Parkinsons Dis 2011;2011:247467. [Crossref] [PubMed]
- Magalingam KB, Radhakrishnan AK, Haleagrahara N. Protective Mechanisms of Flavonoids in Parkinson's Disease. Oxid Med Cell Longev 2015;2015:314560. [Crossref] [PubMed]
- Skoumalová A, Hort J. Blood markers of oxidative stress in Alzheimer's disease. J Cell Mol Med 2012;16:2291-300. [Crossref] [PubMed]
- García-Blanco A, Peña-Bautista C, Oger C, et al. Reliable determination of new lipid peroxidation compounds as potential early Alzheimer Disease biomarkers. Talanta 2018;184:193-201. [Crossref] [PubMed]
- Yoshida Y, Umeno A, Akazawa Y, et al. Chemistry of lipid peroxidation products and their use as biomarkers in early detection of diseases. J Oleo Sci 2015;64:347-56. [Crossref] [PubMed]
- Surendran S, Rajasankar S. Parkinson's disease: oxidative stress and therapeutic approaches. Neurol Sci 2010;31:531-40. [Crossref] [PubMed]
- Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun 2017;482:419-25. [Crossref] [PubMed]
Cite this article as: Taso OV, Philippou A, Moustogiannis A, Zevolis E, Koutsilieris M. Lipid peroxidation products and their role in neurodegenerative diseases. Ann Res Hosp 2019;3:2.


