
The significant characteristics of corticobasal syndrome
Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), dementia is a neurocognitive disorder of the elderly, with various degrees of severity (1). Dementia is caused by a variety of diseases and injuries that primarily or secondarily affect the brain. It is characterized by multiple progressive cognitive deficits resulting in a decline from the previous level of mental function (2). Although the probability of developing dementia rises with age, however, dementia is related to abnormal aging (2,3). The aim of this study was to described symptoms and signs of the CBS and its confirmation with mutation analysis of the related genes.
Historical evolution of corticobasal syndrome (CBS)
CBS is a rare neurodegenerative disorder, which commonly presents with an asymmetrical progressive loss of motor function, which can include apraxia, rigidity, dystonia, and in some cases, alien limb syndrome. CBS was first described by Rebeiz et al. in 1967 (4,5) and termed eventually corticobasal degeneration (CBD). Even though it was considered as a distinct clinicopathologic entity (2,3) clinicopathological studies have revealed that CBD is associated with various clinical syndromes one of which is the CBS, a phenotype of CBD (6,7).
CBS is presumed to be underdiagnosed and the incidence and prevalence of CBS are remaining unknown. The typical onset of the syndrome is between ages 50 and 70 with the most patients do not have a family history of CBS.
Pathology
CBS is associated with a variety of underlying pathologies with CBD pathology represent 50%. More specifically, a wide spectrum of diseases has been associated with CBS, including several proteinopathies such as amyloidopathies, tauopathies, TDP-opathies, synucleinopathies and prionopathies (8-15). The most common associations refer to CBD, are progressive supranuclear palsy (PSP) and Alzheimer’s Disease (AD) pathology. Frontotemporal lobar degeneration with ubiquitin- and TDP-43-positive inclusions (FTLD-TDP), Pick’s disease (Pick), Lewy body disease (LBD), frontotemporal lobar degeneration with fused in sarcoma-positive inclusions (FTLD-FUS) and Creutzfeldt-Jakob disease (CJD) have also linked with CBS (8-15). Biochemical features could only be available after an autopsy as the diagnosis of the underlying cause of CBS is possibly only through postmortem brain analysis. The results of postmortem brain analyses have shown that the majority of causes in CBS are tauopathies. Tau protein exists in six isoforms and different neurodegenerative diseases that can cause CBS, have been associated with specific tau isoforms. CBD and PSP feature predominant disposition of 4R-tau while AD is presented with 3R and 4R-tau protein isoforms, and Pick’s disease with 3R-tau (8-15).
Microscopic findings could also help to differentiate pathologic causes of CBS. Both CBD and PSP presented with threads in gray and white matter but in CBD the boundary between the two of them may be unclear due to the concentration of threads. Astrocytic plaques are the most distinguishing histopathological features between CBD and PSP concerning glia lesions. In PSP the characteristic glia lesions are tufted astrocytes while in CBD astrocytic plaques represent tau accumulation in the distal segments of astrocytes creating a central clear zone. Ballooned neurons are also highly indicative of CBD pathology with swollen cortical neurons often found in third, fifth and sixth layers of Cingulate gyrus, amygdala and insular cortex. Furthermore, oligodendroglial tau inclusions, called coiled bodies, are very frequent in PSP instead of CBD (8-15).
Genetics
Over the last years, there have been numerous advances in descriptions of genetic causes of CBS. However, the genetics of CBS cases is mainly unknown and sporadic. Mutation in the microtubule associated protein tau (MAPT) has been presented in patients with CBS. Also, mutation in the gene that encodes progranulin (PGRN) is frequently observed in CBS patients. In addition, a pathogenetic C9orf72 repeat expansion has associated with CBS, especially in patients with FTD or amyotrophic lateral sclerosis (ALS). With regard to the above-mentioned, patients with MAPT mutations presented with FTLD-tau pathology, while patients with PGRN and C9orf72 mutations with FTLD-TDP pathology. The VCP (valosin-containing protein), CHMP2B (encodes charged multivesicular body protein 2b), TARDBP (TAR DNA-binding protein 43) and FUS (encodes RNA-binding protein FUS) mutations are also associated with FTLD pathology but account for <3% of familial FTLD cases (16). In patients with familial and sporadic FTLD, the microtubule associated protein tau (MAPT), progranulin (PGRN) and C9orf72 mutations presented as the three most common causes of the CBS (17,18).
Clinical signs and symptoms
Clinical progression of CBS is variable. Cortical signs and symptoms are both present in patients with CBS. Patients may demonstrate motor, cognitive or a combination of the two impairments. According to previous researches a predominant frontal and parietal involvement probably leads to these distinct cortical signs and symptoms (19).
Relating to motor presentation, levodopa-unresponsive parkinsonism, asymmetric akinesia and rigidity as well as corticobasal deficits such as dystonia, myoclonus, limb apraxia and alien limb phenomenon are the core features of CBS (20,21). In particular, patients with CBS characterized by an absence or abnormally slow movement (akinesia/bradykinesia), stiffness or resistance to movement (rigidity) and a sustained or a repetitive muscle contractions resulting in twisting movements or abnormal postures (dystonia). In absence of impaired muscle control, affected voluntary positioning and sequencing of muscle movements are also associated with CBS (Table 1).
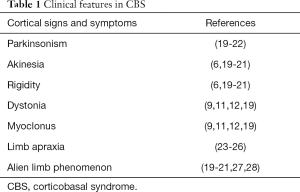
Full table
From a cognitive perspective, CBS is also characterized (27,29) by distinguishing deficits in language, visuospatial dysfunction, and social cognition (3). Several CBS patients faced obvious speech and language problems in the early stage of the syndrome. In particular, patients presented with agraphia (4,23,28), difficulties in spontaneous speech and both single-word and sentence repetition impaired (30). As the syndrome progresses the language dysfunction may be aggravated (27,30,31). Also, most patients demonstrate impaired visuospatial function and it is possible to develop Balint’s syndrome (30,32,33). Inability to express and recognize emotions is observed in patients with CBS and positively correlated with changes in behavior and personality, similar to those observed in FTD (27,30,34). Executive function and memory deficits may also be present in patients with CBS but these deficits are not specific and pathognomonic for CBS in relation with other neurodegenerative diseases, such as frontotemporal dementia, PSP, or AD (3) (Table 2).
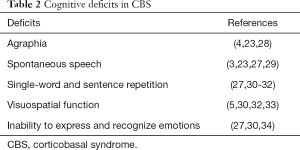
Full table
MRI findings in previous studies
Recent advances in neuroimaging have shed light on specific structural neuroanatomical changes that occur as a result of CBS, even though the pathophysiology of CBS is largely unidentified. Neuroimaging studies with voxel-based morphometry (VBM) have consistently demonstrated atrophy of the gray matter (GM) loss in frontoparietal regions. In particular, primary motor cortex, inferior parietal, left supplementary motor area (24,35-39), and subcortical structures, including the basal ganglia, were presented significantly impaired in CBS patients (3,36,39-42). A more frontal involvement may indicate CBD and TDP-43 pathologies while a more widespread atrophy including post-temporal and parietal areas AD pathology (24,35-39). Furthermore, glucose hypometabolism in the frontal, temporal and parietal lobes, as well as the basal ganglia have been observed in patients with CBS, especially contralateral to the impaired side (22,25,26,43,44) (Table 3).
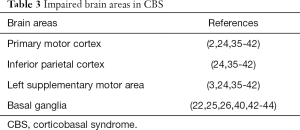
Full table
CBS treatment
CBS treatment includes pharmacologic treatment for motor, cognitive and behavior symptoms of CBS and is symptomatic. The parkinsonism symptoms CBS are treated typically with levodopa, with a slight response. Other treatments such as other dopaminergic therapies, benzodiazepines and anticholinergics have no effect and may be associated with worsened cognition (13,21,45,46). Targeted botulinum toxin injections are used to manage dystonia symptoms (45,47-49). Benzodiazepines, anticholinergic agents, or muscle relaxants are also tried but these are usually ineffective (44,45). Instead, therapeutic strategies for myoclonus include benzodiazepines, particularly clonazepam which is typically the most responsive (22,45,50). Levetiracetam may also be beneficial (18,51). Use of other agents such as gabapentin and valproic acid is also described (50). Cognitive symptoms of CBS are treated with Acetylcholinesterase inhibitors (AChEIs) including rivastigmine, donepezil, galantamine and memantine. However, even though pharmacologic treatment with AChEIs has biological plausibility it remains unidentified and relates to the underlying pathology of CBS (52-58). Patients with CBS have also experienced depression, anxiety and obsessive-compulsive features which can be treated with selective serotonin reuptake inhibitors (SSRIs) (50). Problematic and inappropriate behaviors are treated with atypical neuroleptics or mood-stabilizing agents (50). Apathy is treated with psychostimulants and AChEIs (50), however, the results are controversial (59). Also, cognitive behavior therapies, occupational therapy and kinesiotherapy are addressed in CBS patients in order to improve speech, language and motor symptoms.
Conclusions
The CBS belongs to dementia family syndromes. The main clinical characteristics are levodopa-unresponsive parkinsonism, asymmetric akinesia and rigidity in combination with dystonia, myoclonus, and limb apraxia. The most significant microscopic findings, with regard to differential diagnosis, are threads in gray and white matter, astrocytic plaques concerning glia lesions, ballooned neurons and oligodendroglial tau inclusions. MRI pathognomonic results are as follow: atrophy of gray matter in the primary motor cortex, inferior parietal area, left supplementary motor area and basal ganglia. The therapeutic process is related to many schemas such as levodopa, benzodiazepines, anticholinergics, muscle relaxants, Acetylcholinesterase inhibitors, selective serotonin reuptake inhibitors, atypical neuroleptics and mood-stabilizing agents. The heterogeneity of CBS in pathological, cognitive, behavioral, radiological and clinical characteristics can lead to diagnostic and treatment difficulties.
In the present study, we underline the associated with CBS symptoms and signs in relation to the main mutations which confirm the clinical diagnosis of CBS. The clinical and mutation analysis of the disorder could be the most interesting factor which could mirror the gene therapeutic procedure of the disorder. Differential diagnosis of this disorder could be greatly helped by identification of specific biomarkers in CSF, in relation to mutation analysis of the gene. It may be concluded that clinical symptoms and signs of the suspected patients could be confirmed with mutation analysis.
Acknowledgements
The authors would like to thank the reviewers and editor for the helpful comments on the previous draft.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American P, Association. Neurocognitive Disorders. In: Association AP. editor. Diagnostic and statistical manual of mental disorders 5th ed. Arlington 2013:591-2.
- Cunningham EL, McGuinness B, Herron B, et al. Dementia. Ulster Med J 2015;84:79-87. [PubMed]
- Burrell JR, Hodges JR, Rowe JB. Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord 2014;29:684-93. [Crossref] [PubMed]
- Rebeiz JJ, Kolodny EH, Richardson EP Jr. Corticodentatonigral degeneration with neuronal achromasia: a progressive disorder of late adult life. Trans Am Neurol Assoc 1967;92:23-6. [PubMed]
- Rebeiz JJ, Kolodny EH, Richardson EP Jr. Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 1968;18:20-33. [Crossref] [PubMed]
- Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496-503. [Crossref] [PubMed]
- Hassan A, Whitwell JL, Josephs KA. The corticobasal syndrome-Alzheimer's disease conundrum. Expert Rev Neurother 2011;11:1569-78. [Crossref] [PubMed]
- Boeve BF. Corticobasal degeneration: the syndrome and the disease. In: Litvan I. editor. Atypical parkinsonian disorders. Totawa: Humana Press, 2005:309-34.
- Boeve BF. The multiple phenotypes of corticobasal syndrome and corticobasal degeneration: implications for further study. J Mol Neurosci 2011;45:350-3. [Crossref] [PubMed]
- Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999;53:795-800. [Crossref] [PubMed]
- Ling H, O'Sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 2010;133:2045-57. [Crossref] [PubMed]
- Ouchi H, Toyoshima Y, Tada M, et al. Pathology and sensitivity of current clinical criteria in corticobasal syndrome. Mov Disord 2014;29:238-44. [Crossref] [PubMed]
- Schneider JA, Watts RL, Gearing M, et al. Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 1997;48:959-69. [Crossref] [PubMed]
- Shehata HS, Shalaby NM, Esmail EH, et al. Corticobasal degeneration: clinical characteristics and multidisciplinary therapeutic approach in 26 patients. Neurol Sci 2015;36:1651-7. [Crossref] [PubMed]
- Tartaglia MC, Sidhu M, Laluz V, et al. Sporadic corticobasal syndrome due to FTLD-TDP. Acta Neuropathol 2010;119:365-74. [Crossref] [PubMed]
- Doi H, Tanaka F. The genetics of corticobasal syndrome. Brain Nerve 2013;65:19-30. [PubMed]
- Rohrer JD, Beck J, Warren JD, et al. Corticobasal syndrome associated with a novel 1048_1049insG progranulin mutation. J Neurol Neurosurg Psychiatry 2009;80:1297-8. [Crossref] [PubMed]
- Rossi G, Marelli C, Farina L, et al. The G389R mutation in the MAPT gene presenting as sporadic corticobasal syndrome. Mov Disord 2008;23:892-5. [Crossref] [PubMed]
- Kouri N, Whitwell JL, Josephs KA, et al. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol 2011;7:263-72. [Crossref] [PubMed]
- Gibb WR, Luthert PJ, Marsden CD. Clinical and pathological features of corticobasal degeneration. Adv Neurol 1990;53:51-4. [PubMed]
- Riley DE, Lang AE, Lewis A, et al. Cortical-basal ganglionic degeneration. Neurology 1990;40:1203-12. [Crossref] [PubMed]
- Zhao P, Zhang B, Gao S. 18F-FDG PET study on the idiopathic Parkinson's disease from several parkinsonian-plus syndromes. Parkinsonism Relat Disord 2012;18 Suppl 1:S60-2. [Crossref] [PubMed]
- Leiguarda R, Lees AJ, Merello M, et al. The nature of apraxia in corticobasal degeneration. J Neurol Neurosurg Psychiatry 1994;57:455-9. [Crossref] [PubMed]
- Rohrer JD, Rossor MN, Warren JD. Apraxia in progressive nonfluent aphasia. J Neurol 2010;257:569-74. [Crossref] [PubMed]
- Peigneux P, Salmon E, Garraux G, et al. Neural and cognitive bases of upper limb apraxia in corticobasal degeneration. Neurology 2001;57:1259-68. [Crossref] [PubMed]
- Sakurai Y, Ishii K, Sonoo M, et al. Progressive apraxic agraphia with micrographia presenting as corticobasal syndrome showing extensive Pittsburgh compound B uptake. J Neurol 2013;260:1982-91. [Crossref] [PubMed]
- Kertesz A, Martinez-Lage P, Davidson W, et al. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000;55:1368-75. [Crossref] [PubMed]
- Mathew R, Bak TH, Hodges JR. Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry 2012;83:405-10. [Crossref] [PubMed]
- Kertesz A, McMonagle P. Behavior and cognition in corticobasal degeneration and progressive supranuclear palsy. J Neurol Sci 2010;289:138-43. [Crossref] [PubMed]
- Burrell JR, Hornberger M, Villemagne VL, et al. Clinical profile of PiB-positive corticobasal syndrome. PLoS One 2013;8:e61025. [Crossref] [PubMed]
- Kertesz A, Hudson L, Mackenzie IR, et al. The pathology and nosology of primary progressive aphasia. Neurology 1994;44:2065-72. [Crossref] [PubMed]
- Lang AE, Riley DE, Bergeron C. Cortico-basal ganglionic degeneration In: Calne DB, editor. Neurodegenerative Diseases. Philadelphia: Saunders, 1994:877-94.
- Tang-Wai DF, Josephs KA, Boeve BF, et al. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003;61:1134-5. [Crossref] [PubMed]
- Kluger BM, Heilman KM. Dysfunctional facial emotional expression and comprehension in a patient with corticobasal degeneration. Neurocase 2007;13:165-8. [Crossref] [PubMed]
- Borroni B, Garibotto V, Agosti C, et al. White matter changes in corticobasal degeneration syndrome and correlation with limb apraxia. Arch Neurol 2008;65:796-801. [Crossref] [PubMed]
- Boxer AL, Geschwind MD, Belfor N, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol 2006;63:81-6. [Crossref] [PubMed]
- Josephs KA, Whitwell JL, Boeve BF, et al. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer's disease. Mov Disord 2010;25:1246-52. [Crossref] [PubMed]
- Rinne JO, Lee MS, Thompson PD, et al. Corticobasal degeneration. A clinical study of 36 cases. Brain 1994;117:1183-96. [Crossref] [PubMed]
- Whitwell JL, Jack CR Jr, Boeve BF, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology 2010;75:1879-87. [Crossref] [PubMed]
- Gröschel K, Hauser TK, Luft A, et al. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage 2004;21:714-24. [Crossref] [PubMed]
- Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging 2008;29:280-9. [Crossref] [PubMed]
- Yu F, Barron DS, Tantiwongkosi B, et al. Patterns of gray matter atrophy in atypical parkinsonism syndromes: a VBM meta-analysis. Brain Behav 2015;5:e00329. [Crossref] [PubMed]
- Tripathi M, Dhawan V, Peng S, et al. Differential diagnosis of parkinsonian syndromes using F-18 fluorodeoxyglucose positron emission tomography. Neuroradiology 2013;55:483-92. [Crossref] [PubMed]
- Turaga SP, Mridula R, Borgohain R. Cerebral glucose metabolism, clinical, neuropsychological, and radiological profile in patients with corticobasal syndrome. Neurol India 2013;61:7-11. [Crossref] [PubMed]
- Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998;55:957-61. [Crossref] [PubMed]
- Wenning GK, Litvan I, Jankovic J, et al. Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 1998;64:184-9. [Crossref] [PubMed]
- Cordivari C, Misra VP, Catania S, et al. Treatment of dystonic clenched fist with botulinum toxin. Mov Disord 2001;16:907-13. [Crossref] [PubMed]
- Müller J, Wenning GK, Wissel J, et al. Botulinum toxin treatment in atypical parkinsonian disorders associated with disabling focal dystonia. J Neurol 2002;249:300-4. [Crossref] [PubMed]
- Vanek Z, Jankovic J. Dystonia in corticobasal degeneration. Mov Disord 2001;16:252-7. [Crossref] [PubMed]
- Boeve BF, Josephs KA, Drubach DA. Current and future management of the corticobasal syndrome and corticobasal degeneration. Handb Clin Neurol 2008;89:533-48. [Crossref] [PubMed]
- Kovács T, Farsang M, Vitaszil E, et al. Levetiracetam reduces myoclonus in corticobasal degeneration: report of two cases. J Neural Transm (Vienna) 2009;116:1631-4. [Crossref] [PubMed]
- Boxer AL, Knopman DS, Kaufer DI, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2013;12:149-56. [Crossref] [PubMed]
- Diehl-Schmid J, Forstl H, Perneczky R, et al. A 6-month, open-label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry 2008;23:754-9. [Crossref] [PubMed]
- Hirano S, Shinotoh H, Shimada H, et al. Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain 2010;133:2058-68. [Crossref] [PubMed]
- Kertesz A, Morlog D, Light M, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord 2008;25:178-85. [Crossref] [PubMed]
- Kimura T, Takamatsu J. Pilot study of pharmacological treatment for frontotemporal dementia: risk of donepezil treatment for behavioral and psychological symptoms. Geriatr Gerontol Int 2013;13:506-7. [Crossref] [PubMed]
- Mendez MF, Shapira JS, McMurtray A, et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry 2007;15:84-7. [Crossref] [PubMed]
- Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord 2011;26 Suppl 3:S42-80. [Crossref] [PubMed]
- Armstrong MJ. Diagnosis and treatment of corticobasal degeneration. Curr Treat Options Neurol 2014;16:282. [Crossref] [PubMed]
Cite this article as: Parthimos TP, Schulpis KH. The significant characteristics of corticobasal syndrome. Ann Res Hosp 2019;3:4.


