
A translational approach to the renin-angiotensin-aldosterone system in heart failure
Introduction
The renin-angiotensin-aldosterone system (RAAS) is one of the key human body’s regulatory mechanisms, responsible for maintaining arterial pressure homeostasis, and regulating tissue perfusion. Renin is the rate limiting component of RAAS, while the angiotensin II (AngII) is the active hormone, produced in the extracellular space, after the proteolytic cleavage of the precursor protein (1).
Although renin has been initially described in 1898 as a protein which could increase arterial pressure, RAAS and its role in the pathogenesis of cardiovascular and renal disorders gained more focus during the last decades (2). This is partly attributed to the development of pharmacologic agents effective to block RAAS. This gave the opportunity for an in-depth study of RAAS physiological and pathophysiological roles in experimental setting, as well as their clinical interpretation.
In heart failure (HF) heart fails to adequately fulfill its role as a pump. HF is a syndrome with increased mortality and morbidity drastically affecting the patient’s quality of life (3). Inappropriate activation of RAAS has been indicated as a key pathophysiological mechanism involved in HF as it is directly linked to neurohormonal dysregulation (4). Neurohormonal dysregulation is central in driving disease’s progression. RAAS was the first neurohormonal system that was extensively studied in HF, especially in HF with reduced ejection fraction (HFrEF). This has led to key developments in HF with regard to both understanding HF mechanism, but also in discovering therapeutic strategies for HF patients. It should be noted that although there is robust data on effective therapeutic interventions in HFrEF patients, there is currently no proven effective treatment strategy in HF with preserved ejection fraction (HFpEF) (5). Furthermore, based on the 2016 European Society of Cardiology guidelines for HF, a new group of HF patients with mid-range EF (HFmrEF) has been formed. Patients with HFmrEF have ejection fraction ranging from 40–49% (5). As for HFpEF patients, more data is needed regarding potential underlying pathophysiological mechanisms and therapeutic approaches (5).
In this article we review our current knowledge based on experimental and clinical data on the pathophysiological role of RAAS in HF and we discuss our current treatment options targeting RAAS in HF.
The RAAS from a historical perspective
As previously mentioned, Tigerstedt and Bergmann first published data in 1898 on a substance secreted by renal cortex which is able to increase arterial pressure when injected. This substance was named renin (2). Long time after in 1934, it has been demonstrated that externally-induced renal ischemia, apart from leading to the development of arterial hypertension, also leads to the production of a protein, in addition to renin. Based on further experiments and observations, it has been demonstrated that renin is responsible for the proteolytic cleavage of this protein, which is called angiotensinogen (2). The proteolytic cleavage of the short-lived angiotensinogen, leads to the production of angiotensin. After a number of experiments and purification processes, Ferrario discovered two peptide forms of angiotensin, angiotensin I (AngI) and angiotensin II (AngII) (6). Further, it has been demonstrated that angiotensin-converting enzyme (ACE) mediates AngI cleavage to generate the more active peptide AngII (1). Finally, it has been shown that AngII stimulates the release of hormone aldosterone from the adrenal cortex. Aldosterone has a major role in regulating sodium and potassium balance. Through these mechanisms, RAAS actively participates in controlling blood pressure and electrolyte balance.
RAAS cascade
The juxtaglomerular cells are the key renin producing cells. Various factors could facilitate the production of renin. Renal baroreceptor mechanism in the afferent arteriole could sense even minimal changes in renal perfusion, macula densa cells of the distal tubule sense changes in chloride anion delivery, as well as the activation of sympathetic nervous system through beta-1 receptor activation could facilitate renin production (7). On the other hand, AngII could directly act on juxtaglomerular cells, negatively regulating the production of renin.
Renin is normally produced as a pre-prohormone and after the proteolytic cleavage the mature renin molecule is stored in the juxtaglomerular cells. Upon activation renin is released in the circulation. Renin production and release is the key determinant of RAAS activation. Although angiotensinogen has also been located in various tissues such as kidney, brain heart, placenta and others, liver seems to be the main production source for angiotensinogen (8). Renin cleaves the N-terminal part of angiotensinogen in order to form AngI which consists of 10 peptides. AngII is produced after the C-terminal dipeptide is removed from AngI by ACE (9). ACE is a membrane-bound enzyme which could be found in various cell types, such as endothelial cells. Beyond AngI, ACE also catalyzes the enzyme degradation of other vasoactive peptides, such as bradykinin, to inactive metabolites thus leading to increased vasoconstriction.
AngII as the active final product of the RAAS is the key facilitator of the most significant biological actions of the RAAS. Beyond AngII, there are two other significant metabolites, namely AngIII and AngIV, which are formed from AngII. They are mainly localized in kidney and heart demonstrating actions such as blood pressure regulation (10). Most of these actions are mediated through the binding of AngII to the angiotensin type I receptor (AT1), although there are four distinct angiotensin receptors (11). The key cardiovascular actions mediated by AngII through its binding to AT1 are vasoconstriction, increasing of arterial blood pressure, increasing of cardiac contractility and cardiac hypertrophy. Experimental data also demonstrated that AT1 after binding AngII also mediates effects on cell proliferation, cell growth as well as inflammation and oxidative stress (6). In addition, AT2 receptor seems to play a significant role in cardiac remodeling also exerting anti-proliferative actions in cells such as smooth muscle cells partly through facilitating cell apoptosis (11). It is important to mention that both AngI and AngII are produced very close to their site of action, while ACE is also membrane bound linked to vascular endothelium. Taking these observations into account, renin is the main circulatory hormone in RAAS.
Furthermore, AngII mediates renal tubular sodium reabsorption, regulation of renin release and aldosterone production from the adrenal cortex. Aldosterone plays a significant role in regulating extracellular volume as it directly regulates sodium and potassium balance (12). Specifically, aldosterone acting on the distal tubules increases the reabsorption of sodium and water, which results in increased potassium excretion. Therefore extracellular potassium levels along with AngII expression levels are the most significant regulators of aldosterone production.
Experimental data indicates that renin and prorenin could directly participate in cellular growth processes via their binding to specific (pro-) renin receptors (13). The activation of these receptors via binding prorenin or renin lead to the local production of AngI, but importantly they also lead to the activation of several intracellular signaling pathways. It has been demonstrated that the activation of these receptors can facilitate the phosphorylation of mitogen activated prorenin kinases (MAPKs), such as the extracellular-signal-regulated kinases 1&2 (ERK 1& ERK2) which are linked to transforming the expression of growth factor β1 (TGF-β1) independently of AngII (13). Activation of renin receptor leads to the activation of hypertrophic and apoptotic activities, linked to extracellular matrix remodeling which is crucial for the deterioration of cardiac function, especially in disease states such as HF (14). Prorenin receptor overexpression by using adenovirus-mediated gene delivery led to worsening of cardiac function and activation of myocardial fibrosis (14). These results further supported the hypothesis that potent renin-angiotensin-system blockade in patients with HF could exert antifibrotic actions.
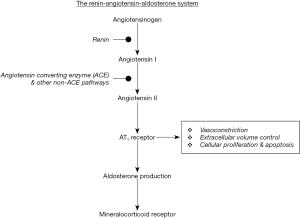
To sum up, the reduction in renal perfusion pressure and afferent arteriolar pressure is the trigger that leads to the release of renin from the juxtaglomerular cells. This reduction could be the result of a temporary decrease in circulating blood volume. Beyond renal perfusion pressure, sympathetic nervous system activation, as well as the reduction of sodium chloride concentration in distal tubules, sensed by macula dense cells, leads to the additional release of renin. Renin catalyzes the formation of AngI via cleaving the N-terminal peptides of circulating angiotensinogen. Consequently, ACE cleaves the C-terminal octapeptide of AngI, leading to the formation of AngII. After its production and the subsequent production of aldosterone, AngII mediates various actions through its binding to AT1 receptor aiming at maintaining homeostasis. AngII via mediating the reabsorption of sodium, as well as renal and systemic vasoconstriction, results in increasing blood pressure, circulating volume and restoring renal perfusion (Figure 1). This leads to gradual reduction in renin release, which negatively regulates RAAS activation. However, it should be noted that under specific conditions or diseases, such as HF, an inappropriate activation of RAAS with detrimental effects has been observed.
RAAS activation in cardiovascular diseases
Hypertension is one of the most studied cardiovascular disorders in which RAAS activation seems to play a pivotal role. RAAS dysregulation seems to be involved both in essential and in secondary hypertensive disorders (15,16). It has been demonstrated that significant number of patients with essential hypertension have moderately increased plasma renin activity. This has been partly attributed to increased sympathetic activity. On the other hand, most patients with hypertension have normal renin levels. However, it has been speculated that normal renin levels in hypertensive patients may be a sign of RAAS dysregulation. More specifically, it has been demonstrated that RAAS blockade has a therapeutic effect in hypertensive patients with normal renin levels, indirectly indicating the possibly inappropriate activation of RAAS and renin in this group of patients. Moreover more than 25% of hypertensive patients have low circulating renin levels. This is partly expected given the negative feedback that increased blood pressure could have on RAAS activation. Low renin levels in hypertensive patients are also linked to sodium and volume overload. Beyond hypertension, there is data demonstrating the role of RAAS dysregulation in primary aldosteronism. It should be noted that hypertensive patients with high renin levels are in mostly male and of young age. Hypertensive patients with low renin levels are in most cases African-American women, while diabetes type 2 has also been linked with low renin levels (17).
Alongside with hypertension, HF is the cardiovascular disorder in which RAAS dysregulation is of vital importance.
RAAS in HF
Intensive ongoing research efforts are made in order to discover the ways RAAS dysregulation affect the progression of HF, but more importantly how the existing or newly developed RAAS blocking agents could be effectively used in order to optimally control the detrimental effects of the disease and RAAS dysregulation.
Experimental data has demonstrated that increased cardiac wall stress could induce the production of angiotensinogen. Angiotensinogen mRNA levels were increased in cardiac hypertrophy, specifically in animal models of pressure overload cardiac hypertrophy (18-20). Furthermore, there has been observed a significant increase in angiotensinogen gene expression from cardiomyocytes in rat animal model with left ventricular failure after coronary artery ligation. These observations indicate the key role that the local production of angiotensinogen could play in cardiac hypertrophy and failure (18-20). However, it should be noted that coronary artery ligation is also linked to ischemia-related activation of intracellular pathways, beyond hypertrophic changes. Similarly, local cardiomyocyte renin mRNA is increased in rats’ infarcted left ventricle (21). It should be noted that cardiomyocyte renin mRNA is generally low, but it seems that renin expression is increased under pathologic conditions (21,22). Experimental data has also demonstrated that cardiomyocytes could produce low renin levels, as it was mainly shown in cardiomyocytes isolated by animal models (23). Renin does not own a direct biologic action. However, as previously mentioned, renin receptor which binds both renin and prorenin induces the activation of intracellular pathways, such as the activation of ERK1 & ERK2, that may result in increased production of AngII, as it was showed in cell culture experiments in rat cardiac myocytes and fibroblasts (24). Given that renin receptor has also been localized in the subendothelial layer of coronary arteries, these observations from cell culture experiments led to the hypothesis§ that renin receptor could partly contribute to the production of angiotensin in the heart (24).
AngII represents the most active component of RAAS. Systemic actions of AngII in disease states such as hypertension and cardiac hypertrophy have already been mentioned. Importantly, it has been demonstrated that AngII could directly affect cardiac metabolism and energetics in HF (25,26). Specifically, it has been shown that increased AngII levels result in a gradual increase in fatty acid β-oxidation and decrease in carbohydrate oxidation, as it was mainly demonstrated in mice treated with AngII (27). Impaired glucose oxidation has been observed in HF, in cardiac hypertrophy, as well as in animal models with dilated cardiomyopathy (28). This AngII-mediated shift in cardiac metabolism could lead further to increased ROS production and mitochondrial dysfunction. This mitochondrial dysfunction and impaired energy metabolism has been linked to both systolic and diastolic dysfunction in experimental models with increased AngII levels, subtly contributing to HF progression (28). As previously mentioned, the animal models used in these experiments were mainly treated with exogenous AngII, which could act differently compared to the endogenous produced AngII in patients with HF.
Increased myocardial expression of ACE has been identified in experimental models of ventricular hypertrophy and in rats’ myocardium after myocardial infarction (29-31). Similarly, increased expression of ACE has been found in myocardium of patients with end-stage HF (31). Furthermore, ACE expression was increased in the scar tissue and the cardiac myocytes from patients after myocardial infarction. In this group ACE increased expression was found in fibroblasts but also endothelial cells, isolated from cardiac tissue obtained at left ventricular aneurysmectomy, compared to the cells obtained from apparently healthy subjects at necropsy (32). On the other hand, evidence suggests that ACE might not be the key enzyme catalyzing the conversion of AngI to AngII in cardiac tissue. Experimental data demonstrate that ACE inhibitors could only block 20% of AngI converted to AngII (33). Further studies are needed in order to better elucidate the role of local expressed ACE in HF.
Angiotensin receptors also play a key role in HF pathophysiology. In vitro data suggest that that rats’ cardiac myocytes demonstrate increased density of AT1 receptor after myocardial infarction. This could be linked to an increased susceptibility of the infarcted myocardium to AngII actions (34), which might be partly mediated by the nuclear-factor kappa B (NF-κB) pathway. Blocking this pathway results in reduced expression of AT1 receptor in rats with chronic HF (35). On the other hand, experimental data from failing human hearts has shown reduced AT1 mRNA expression and a stable or unchanged AT2 mRNA expression (36). AT2 receptor acting via signaling pathways, including the activation of various phosphatases that mediate the inactivation of MAPKs, such as the p42 and p44 MAPKs, could exert beneficial effects in the failing heart (37). Various data demonstrate that AT2 receptor activation under pathological condition could inhibit abnormal cellular growth and cardiac remodeling, while it could also induce vasodilation (38,39). Furthermore, it has been demonstrated that in vitro overexpression of AT2 receptor could attenuate cardiomyocyte fibrosis independently of hypertrophy (40). In addition, mice AT2 receptor overexpression had a more favorable post-MI cardiac remodeling with a preserved left ventricular function (40).
Beyond the most known RAAS components and AT receptors, a number of molecules related to RAAS, having a role in HF, have been discovered. ACE2 is a recently discovered enzyme similar to ACE (41). Experimental data demonstrate that ACE2 activity is increased after MI induction (42). ACE2 cardiac gene and protein expression are increased in patients with HF (42). Interestingly, ACE2 gene ablation led to ventricular dysfunction and dilatation, as well as increased AngII levels (43). Ang-(Jeny1-7) is an AngII fragment that could be formed from AngI via the action of several peptidases. Ang-(Jeny1-7) seems to exert beneficial actions in cardiovascular system. It has been demonstrated that Ang-(Jeny1-7) plays a key role in preserving systolic cardiac function in pressure-overload induced HF model (44). There is evidence indicating that Ang-(Jeny1-7) levels are increased after RAAS inhibition via ACE or ARB, while exogenous administration of Ang-(Jeny1-7) improved cardiac function and reversed cardiac remodeling after MI mainly by reversing the deleterious effects of MI on transforming-growth-factor betta-1 (TGF-β1) induced fibrosis (44,45). Ang-(Jeny1-7) has also anti-inflammatory properties as could inhibit NAPDH oxidase, the enzyme playing pivotal role in ROS generation (46) while also inhibits cell proliferation by inhibiting MAPKs. It has also been demonstrated that Ang-(Jeny1-7) is among the peptides partly mediating the anti-inflammatory and antifibrotic properties of telmisartan or olmesartan (47,48). Finally, it has been shown that Ang-(Jeny1-7) has antiarrhythmic properties via reducing interstitial fibrosis, MAPKs signaling, and by reducing the expression of inflammatory cytokines (49). Ang (Jeny1-9) is also an enzymic fragment derived by the degradation of AngI by ACE2. One the first experimental studies demonstrated that Ang (Jeny1-9) levels were increased after MI induced by coronary artery ligation in rats at first week alongside with the levels of AngII, ACE and ACE2 (50). However, Ang (Jeny1-9) levels were lower at 8th week of follow-up compared to control group, while treatment with ACEi preserved Ang (Jeny1-9) levels. Based on these observations it has been hypothesized that Ang (Jeny1-9) could also act via negative feedback loop counteracting the negative effects of AngII, mediating the beneficial effects of ACEi (50). There is also experimental data demonstrating that Ang (Jeny1-9) could improve, both in vitro and in vivo, the endothelial function mainly via increasing NO bioavailability (51).
RAAS inhibition in HF with reduced ejection fraction
Angiotensin-converting enzyme inhibitors (ACEi)
Angiotensin converting enzyme inhibitors (ACEi) still remain the first line treatment in patients with HF and reduced ejection fraction. Large clinical trials such as the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) and the Studies of Left Ventricular Dysfunction (SOLVD) demonstrated that patients with HFrEF have significantly reduced mortality (52-54). In the CONSENSUS trial treatment of patients with NYHA IV HFrEF with enalapril led to 31% mortality reduction after one year of treatment compared to placebo. SOLVD trials included patients with mild to moderate HF (SOLVD treatment) and patients with asymptomatic, NYHA I, HF (SOLVD prevention) (52,55). In SOLVD treatment trial enalapril treatment led to significant decrease of morbidity and mortality. Furthermore, in the group of asymptomatic patients enalapril seemed to significantly reduce the progression of the disease. Similar were the results for patients with left ventricular dysfunction after MI. Numerous data support the positive effect of ACEi on LV remodeling and functionality after MI or in patients with HFrEF. The Survival and Ventricular Enlargement (SAVE) and the Trandolapril Cardiac Evaluation (TRACE) trials demonstrated that ACEi treatment could significantly slow the development of HF after MI (54,56). ACEi by inhibiting the conversion of AngI to AngII seems to positively affect the neurohormonal state in HF patients. This along with other pleiotropic effects of ACEi on cellular processes such as fibrosis, apoptosis seem to be the underlying mechanisms mediating the role of ACEi inhibiting the remodeling process (57-60).
Angiotensin receptor blockers (ARBs)
After their development, ARBs were supposed to offer a more effective RAAS blockade given their effect on angiotensin receptor. Compared to ACEi, ARBs do not inhibit bradykinin breakdown. As increased bradykinin levels are linked to airway irritation, cough, and increased risk for angioedema, ARBs are better tolerated compared to ACEi. It has been hypothesized that ARBs via inhibiting the effects of AngII on its receptor could be more effective compared to ACEi. However, ARBs showed non-inferiority but not superiority to ACEi in HF clinical trials. In one of the first studies the Evaluation of Losartan in the Elderly II (ELITE II) study, losartan failed to show superiority compared to captopril, although there was much of discussion that the dose of losartan used was not optimal (60). The HF Endpoint evaluation of Angiotensin II Antagonist Losartan (HEAAL) trial demonstrated the superiority of the 150 mg/day losartan dose compared with the 50 mg/day in reducing mortality risk and HF hospitalizations, in HF patients with ACEi intolerance (61). In addition, the Candesartan in Heart Failure-Assessment of Reduction in Mortality and morbidity (CHARM-Alternative) trial further demonstrated that treatment with candesartan led to significantly reduced cardiovascular mortality or HF hospitalizations in HF patients with ACEi intolerance (62). Further trials tried to study the combined effect of ACEi and ARBs. Clinical studies and meta-analyses demonstrated that the combination of ACEi and ARBs could result in reduced risk for HF hospitalizations, but they had no significant additional benefit on HF mortality risk. The Valsartan Heart Failure Trial (Val-HeFT) has shown that the combination of ACEi with valsartan had no effect on mortality risk compared to placebo, but led to less hospitalizations (63). Similarly, ACEi combined with ARBs had no additional benefit for patients with acute MI in the Valsartan In Acute myocardial Infarction (VALIANT) trial or for patients with stable CAD in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) (64,65). On the other hand, various analyses demonstrated that the ACEi and ARBs combination could increase the risk for hypotension or acute kidney injury and hyperkaliemia.
Mineralocorticoid receptor antagonists (MRAs)
One of the interesting findings in HF patients who are under chronic treatment with ACEi is the aldosterone escape phenomenon (66). It has been observed that there is a number of HF patients treated with ACEi that have increased aldosterone levels. Aldosterone results in increased water and salt retention. This attributed to the fact that ACEi although reduce AngII also increase potassium levels which lead to increased aldosterone production. Furthermore, experimental data demonstrated that aldosterone induce myocardial fibrosis which has a detrimental effects on ventricular remodeling in HF (67). Both experimental and clinical data showed the beneficial effects of aldosterone blockade in HF. The Randomized Aldactone Evaluation Study (RALES) is one of the first studies that investigated the role of spironolactone therapy added to ACEi in patients with HFrEF and NYHA class IV symptoms (67). In the RALES study patients were randomized to receive 25 mg of spironolactone versus placebo, while the dose could be increased up to 50 mg/day. Notably, the study was early terminated due to a significant reduction of mortality risk compared to placebo (67). Spironolactone significantly reduced both the risk for sudden cardiac death and deaths due to HF progression. Eplerenone is a more specific inhibitor mineralocorticoid receptor compared to spironolactone. The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) investigated the role of eplerenone in patients with left ventricular dysfunction and HF after myocardial infarction (68). Study results demonstrate that eplerenone significantly reduced cardiovascular death and hospitalizations due to HF, as well as total mortality in this group of patients (68). Similarly, the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS- HF) demonstrated similar results in patients with NYHA II–IV HF treated with eplerenone (69). A number of pathophysiologic mechanisms are involved in mediating the positive actions of aldosterone blockers in patients with HF. It has been shown that MRAs improve endothelial function, while aldosterone blockade may reduce the risk for ventricular arrhythmias. The antiarrhythmic effect of aldosterone blockade may be attributed to more balanced potassium levels or to the antifibrotic effects of MRAs (69). Finally, it has been demonstrated that overexpression of aldosterone receptors leads to ventricular arrhythmias, thus aldosterone blockade might me linked to decreased risk for ventricular arrhythmias (70).
Direct renin inhibitors
It is known that RAAS blockade via ACEi or ARBs may be limited due to the remaining increased renin activity. Both ACEi and ARBs reduce AngII production, thus limiting the negative feedback of AngII on renin release. As mentioned earlier, renin and prorenin could induce AngII production via the chymase and cathepsin pathways, while they can also induce the production of other angiotensin subtypes upstream to the RAAS blockade, such as angiotensin III or angiotensin 1-7. Furthermore, renin receptor further mediates significant actions of renin and prorenin despite RAAS blockade. In order to further block the aforementioned renin action, direct renin inhibitors were developed. Aliskiren represents the most active and efficient direct renin inhibitor tested in HF (71). Aliskiren blocks the active site of renin and prorenin, while it could block both circulating and tissue renin (71). As for ACEi and ARBs, aliskiren owes a blood-pressure lowering ability comparable to the aforementioned RAAS blockers categories (72). Based on the initial results from proof-of-concept trial data, aliskiren resulted in greater reduction in renin, AngII and aldosterone levels in HF patients, compared to Ramipril (73). However, major concerns have been raised due to the significant adverse effects of aliskiren in diabetic patients, as well as in patients with impaired kidney function (74).
Angiotensin receptor-neprilysin inhibitors (ARNIs)
Combined ARNIs represent a novel development in the HFrEF treatment. It is known that natriuretic peptides (NPs) are vasoactive released in response to the reactive activation of RAAS and sympathetic nervous system in HFrEF, due to the abnormal distension of atria and ventricles. Atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) are the most common NPs (75). Experimental data demonstrated that NPs have antifibrotic and anti-hypertrophic properties, beyond their vasodilatory role in HF (75). Based on these findings, it has been hypothesized that NPs could serve as a potential therapeutic target in HF.
Neprilysin is a membrane bound endopeptidase catalyzing NPs degradation (76). Beyond NPs, neprilysin also catalyzes the degradation of various substances, such as bradykinin, substance-P, adrenomedullin and other vasoactive substances (76). Therefore, neprilysin inhibition was considered a potential therapeutic target in HF. Sacubitril/valsartan represents the first ARNI officially approved to be used in HFrEF patients. Sacubitril upon ingestion is metabolized into sacubitrilat which is an active neprilysin inhibitor. The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) was a phase III randomized clinical trial which compared sacubitril/ valsartan with enalapril in HFrEF patients (77). It was found that sacubitril/ valsartan significantly reduced the primary composite endpoint of cardiovascular death and HF hospitalization in patients with chronic HF (77). A number of clinical studies further investigate the role of sacubitril/ valsartan in various HF patient groups, such as HFpEF patients and patients with HF after MI (78,79).
Conclusions
RAAS plays a significant role in regulating blood pressure and fluid volume balance participating in human body’s homeostasis. Numerous experimental studies tried to study the role of circulating and tissue-localized hormones of RAAS. Dysregulation of RAAS has been indicated in various cardiovascular diseases such as hypertension and HF. Understanding the pathophysiology and the role of RAAS in HF led to the development of specific therapeutic interventions targeting RAAS. ACE inhibitors are still considered the gold standard for HFrEF therapy, while ARBs in most cases are used in patients with ACEi intolerance. Aldosterone antagonists have also demonstrated a benefit on mortality of HFrEF patients through a number of pleiotropic actions. Recent data also indicated an additional morbidity and mortality benefit for sacubitril/ valsartan, an angiotensin receptor-neprilysin inhibitor, in HFrEF. Although clinical data established the use of the aforementioned therapies in HFrEF, there is no proven therapy for patients with HFmrEF and HFpEF. Further studies are needed in order to define the role of RAAS in HFpEF, and the optimal therapeutic strategy for this group of patients.
Acknowledgments
We thank Dr. Ioanna Bakogianni for her help on the revised version of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Skeggs LT Jr, Kahn JR, Lentz K, et al. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med 1957;106:439-53. [Crossref] [PubMed]
- Piepho RW, Beal J. An overview of antihypertensive therapy in the 20th century. J Clin Pharmacol 2000;40:967-77. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med 2010;362:228-38. [Crossref] [PubMed]
- Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011;32:670-9. [Crossref] [PubMed]
- Ferrario CM. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst 2006;7:3-14. [Crossref] [PubMed]
- Brown MJ. Renin: friend or foe? Heart 2007;93:1026-33. [Crossref] [PubMed]
- Morgan L, Broughton Pipkin F, Kalsheker N. Angiotensinogen: molecular biology, biochemistry and physiology. Int J Biochem Cell Biol 1996;28:1211-22. [Crossref] [PubMed]
- Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 2003;24:261-71. [Crossref] [PubMed]
- Reudelhuber TL. The renin-angiotensin system: peptides and enzymes beyond angiotensin II. Curr Opin Nephrol Hypertens 2005;14:155-9. [Crossref] [PubMed]
- Stanton A. Now that we have a direct renin inhibitor, what should we do with it? Curr Hypertens Rep 2008;10:194-200. [Crossref] [PubMed]
- Funder JW. Aldosterone and Mineralocorticoid Receptors-Physiology and Pathophysiology. Int J Mol Sci 2017.18. [PubMed]
- Nguyen G. The (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Curr Opin Nephrol Hypertens 2007;16:129-33. [Crossref] [PubMed]
- Moilanen AM, Rysa J, Serpi R, et al. (Pro)renin receptor triggers distinct angiotensin II-independent extracellular matrix remodeling and deterioration of cardiac function. PLoS One 2012;7:e41404. [Crossref] [PubMed]
- Te Riet L, van Esch JH, Roks AJ, et al. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 2015;116:960-75. [Crossref] [PubMed]
- Laragh J. Laragh's lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens 2001;14:837-54. [Crossref] [PubMed]
- Monticone S, Losano I, Tetti M, et al. Diagnostic approach to low-renin hypertension. Clin Endocrinol (Oxf) 2018;89:385-96. [Crossref] [PubMed]
- Baker KM, Chernin MI, Wixson SK, et al. Renin-angiotensin system involvement in pressure-overload cardiac hypertrophy in rats. Am J Physiol 1990;259:H324-32. [PubMed]
- Lindpaintner K, Lu W, Neidermajer N, et al. Selective activation of cardiac angiotensinogen gene expression in post-infarction ventricular remodeling in the rat. J Mol Cell Cardiol 1993;25:133-43. [Crossref] [PubMed]
- Finckh M, Hellmann W, Ganten D, et al. Enhanced cardiac angiotensinogen gene expression and angiotensin converting enzyme activity in tachypacing-induced heart failure in rats. Basic Res Cardiol 1991;86:303-16. [Crossref] [PubMed]
- Passier RC, Smits JF, Verluyten MJ, et al. Expression and localization of renin and angiotensinogen in rat heart after myocardial infarction. Am J Physiol 1996;271:H1040-8. [PubMed]
- Danser AH, van Kats JP, Admiraal PJ, et al. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension 1994;24:37-48. [Crossref] [PubMed]
- Re RN. The clinical implication of tissue renin angiotensin systems. Curr Opin Cardiol 2001;16:317-27. [Crossref] [PubMed]
- van Kesteren CA, Danser AH, Derkx FH, et al. Mannose 6-phosphate receptor-mediated internalization and activation of prorenin by cardiac cells. Hypertension 1997;30:1389-96. [Crossref] [PubMed]
- Lopaschuk GD, Ussher JR, Folmes CD, et al. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207-58. [Crossref] [PubMed]
- Mori J, Basu R, McLean BA, et al. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail 2012;5:493-503. [Crossref] [PubMed]
- Lewis GD, Asnani A, Gerszten RE. Application of metabolomics to cardiovascular biomarker and pathway discovery. J Am Coll Cardiol 2008;52:117-23. [Crossref] [PubMed]
- Turer AT. Using metabolomics to assess myocardial metabolism and energetics in heart failure. J Mol Cell Cardiol 2013;55:12-8. [Crossref] [PubMed]
- Schunkert H, Dzau VJ, Tang SS, et al. Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. Effects on coronary resistance, contractility, and relaxation. J Clin Invest 1990;86:1913-20. [Crossref] [PubMed]
- Challah M, Nicoletti A, Arnal JF, et al. Cardiac angiotensin converting enzyme overproduction indicates interstitial activation in renovascular hypertension. Cardiovasc Res 1995;30:231-9. [Crossref] [PubMed]
- Wollert KC, Studer R, von Bulow B, et al. Survival after myocardial infarction in the rat. Role of tissue angiotensin-converting enzyme inhibition. Circulation 1994;90:2457-67. [Crossref] [PubMed]
- Hokimoto S, Yasue H, Fujimoto K, et al. Increased angiotensin converting enzyme activity in left ventricular aneurysm of patients after myocardial infarction. Cardiovasc Res 1995;29:664-9. [Crossref] [PubMed]
- Urata H, Healy B, Stewart RW, et al. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res 1990;66:883-90. [Crossref] [PubMed]
- Meggs LG, Coupet J, Huang H, et al. Regulation of angiotensin II receptors on ventricular myocytes after myocardial infarction in rats. Circ Res 1993;72:1149-62. [Crossref] [PubMed]
- Guggilam A, Cardinale JP, Mariappan N, et al. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol 2011;106:273-86. [Crossref] [PubMed]
- Haywood GA, Gullestad L, Katsuya T, et al. AT1 and AT2 angiotensin receptor gene expression in human heart failure. Circulation 1997;95:1201-6. [Crossref] [PubMed]
- Ichiki T, Labosky PA, Shiota C, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 1995;377:748-50. [Crossref] [PubMed]
- Ichihara S, Senbonmatsu T, Price E Jr, et al. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation 2001;104:346-51. [Crossref] [PubMed]
- Senbonmatsu T, Ichihara S, Price E Jr, et al. Evidence for angiotensin II type 2 receptor-mediated cardiac myocyte enlargement during in vivo pressure overload. J Clin Invest 2000;106:R25-9. [Crossref] [PubMed]
- AbdAlla S. Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 2000;407:94-8. [Crossref] [PubMed]
- Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275:33238-43. [Crossref] [PubMed]
- Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417:822-8. [Crossref] [PubMed]
- Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87:E1-9. [Crossref] [PubMed]
- Patel VB, Bodiga S, Fan D, et al. Cardioprotective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1-7 in experimental heart failure in angiotensin-converting enzyme 2-null mice. Hypertension 2012;59:1195-203. [Crossref] [PubMed]
- Marques FD, Ferreira AJ, Sinisterra RD, et al. An oral formulation of angiotensin-(1-7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension 2011;57:477-83. [Crossref] [PubMed]
- Shenoy V, Grobe JL, Qi Y, et al. 17beta-Estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats. Peptides 2009;30:2309-15. [Crossref] [PubMed]
- Sukumaran V, Veeraveedu PT, Gurusamy N, et al. Cardioprotective effects of telmisartan against heart failure in rats induced by experimental autoimmune myocarditis through the modulation of angiotensin-converting enzyme-2/angiotensin 1-7/mas receptor axis. Int J Biol Sci 2011;7:1077-92. [Crossref] [PubMed]
- Sukumaran V, Veeraveedu PT, Lakshmanan AP, et al. Olmesartan medoxomil treatment potently improves cardiac myosin-induced dilated cardiomyopathy via the modulation of ACE-2 and ANG 1-7 mas receptor. Free Radic Res 2012;46:850-60. [Crossref] [PubMed]
- Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension 2001;38:665-8. [Crossref] [PubMed]
- Ocaranza MP, Lavandero S, Jalil JE, et al. Angiotensin-(1-9) regulates cardiac hypertrophy in vivo and in vitro. J Hypertens 2010;28:1054-64. [Crossref] [PubMed]
- Flores-Munoz M, Work LM, Douglas K, et al. Angiotensin-(1-9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension 2012;59:300-7. [Crossref] [PubMed]
- Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302. [Crossref] [PubMed]
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429-35. [Crossref] [PubMed]
- Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327:669-77. [Crossref] [PubMed]
- Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685-91. [Crossref] [PubMed]
- Køber L, Torp-Pedersen C, Carlsen J, et al. An echocardiographic method for selecting high risk patients shortly after acute myocardial infarction, for inclusion in multi-centre studies (as used in the TRACE study). TRAndolapril Cardiac Evaluation. Eur Heart J 1994;15:1616-20. [Crossref] [PubMed]
- Elder DH, Lang CC, Choy AM. Pacing-induced heart disease: understanding the pathophysiology and improving outcomes. Expert Rev Cardiovasc Ther 2011;9:877-86. [Crossref] [PubMed]
- Elder DH, Lang CC, Rekhraj S, et al. Renin-angiotensin system blockers are associated with reduced mortality and heart failure hospitalization in patients paced for complete atrioventricular block. Heart Rhythm 2012;9:505-10. [Crossref] [PubMed]
- Goussev A, Sharov VG, Shimoyama H, et al. Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am J Physiol 1998;275:H626-31. [PubMed]
- Pahor M, Bernabei R, Sgadari A, et al. Enalapril prevents cardiac fibrosis and arrhythmias in hypertensive rats. Hypertension 1991;18:148-57. [Crossref] [PubMed]
- Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet 2009;374:1840-8. [Crossref] [PubMed]
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772-6. [Crossref] [PubMed]
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75. [Crossref] [PubMed]
- Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002;360:752-60. [Crossref] [PubMed]
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-906. [Crossref] [PubMed]
- Zannad F. Angiotensin-converting enzyme inhibitor and spironolactone combination therapy. New objectives in congestive heart failure treatment. Am J Cardiol 1993;71:34A-9A. [Crossref] [PubMed]
- Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann N Y Acad Sci 2002;970:89-100. [Crossref] [PubMed]
- Pitt B, Bakris G, Ruilope LM, et al. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008;118:1643-50. [Crossref] [PubMed]
- Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11-21. [Crossref] [PubMed]
- Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation 2005;111:3025-33. [Crossref] [PubMed]
- Vaidyanathan S, Jarugula V, Dieterich HA, et al. Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clin Pharmacokinet 2008;47:515-31. [Crossref] [PubMed]
- Gradman AH, Kad R. Renin inhibition in hypertension. J Am Coll Cardiol 2008;51:519-28. [Crossref] [PubMed]
- Pitt B, Latini R, Maggioni AP, et al. Neurohumoral effects of aliskiren in patients with symptomatic heart failure receiving a mineralocorticoid receptor antagonist: the Aliskiren Observation of Heart Failure Treatment study. Eur J Heart Fail 2011;13:755-64. [Crossref] [PubMed]
- Jhund PS, McMurray JJ, Chaturvedi N, et al. Mortality following a cardiovascular or renal event in patients with type 2 diabetes in the ALTITUDE trial. Eur Heart J 2015;36:2463-9. [Crossref] [PubMed]
- Mangiafico S, Costello-Boerrigter LC, Andersen IA, et al. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J 2013;34:886-93c. [Crossref] [PubMed]
- Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016;102:1342-7. [Crossref] [PubMed]
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004. [Crossref] [PubMed]
- von Lueder TG, Wang BH, Kompa AR, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 2015;8:71-8. [Crossref] [PubMed]
- Solomon SD, Rizkala AR, Gong J, et al. Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial. JACC Heart Fail 2017;5:471-82. [Crossref] [PubMed]
Cite this article as: Bakogiannis C, Theofilogiannakos E, Papadopoulos C, Lazaridis C, Bikakis I, Tzikas S, Vassilikos V. A translational approach to the renin-angiotensin-aldosterone system in heart failure. Ann Res Hosp 2019;3:11.


