
Novel insight on the impact of choline-deficiency in sepsis
Introduction
Choline is a necessary dietary component that maintains normal function and integrity of the body cells (1-3). It is a biological molecule presented in tissues as phosphatidylcholine (PtdCho) or sphingomyelin (SM). Choline is a major donor for methyl groups essential, among others, for DNA methylation and repair, signaling pathways, lipid and cholesterol transport, and metabolism. The important choline metabolites include acetylcholine (ACh), platelet-activating factor, lysophosphatidylcholine (LysoPC), phosphocholine (Pho), glycerophosphocholine (GPCho), plasmalogens, and betaine (2).
Choline has been officially approved by the US Food and Nutrition Board as an essential nutrient for the maintenance of health and the human adequate dietary intake of choline has been addressed (4). Choline is available in the diet either in a free form or in a bound form (esters) which represent most of the body stores of each, such as Pho, GPCho, SM or PtdCho (2). Dietary choline is absorbed by the small intestine and its uptake is mediated by specific choline transporters in the intestine, where eventually it is converted into PtdCho (also known as lecithin) (5); PtdCho is the main phospholipid (>50%) in the mammalian cell membrane (3). The various forms of choline have different bioavailability; the ester form of choline enters via lymph and bypasses the liver (6), while the free form of choline enters the portal circulation and is mostly uptaken by the liver (7). Choline plays a major role in the production of the essential amino acid methionine from homocysteine via the choline derivative betaine (8); it has an important metabolic role in different organs and tissues including brain, liver, kidneys, placenta and mammary glands. Choline can be synthesized endogenously by the liver via de novo mechanism as well. Although the de novo mechanism catalyzed by the liver enzyme phosphatidylethanolamine-N-methyltransferase (PEMT) to produce new moiety of choline in the form of PtdCho compensates for the lack of dietary choline, the endogenous biosynthesis of choline is considered insufficient to meet all human requirements for choline (9).
The recommended adequate intake for choline is 425 mg/day for women, 450 mg/day for pregnant women, 550 mg/day for men and lactating women as well. Nevertheless, several factors like genetic makeup, age, gender, menopausal status, ethnic and racial backgrounds of an individual affect choline metabolism and subsequent choline requirements (4,10). Therefore, the risk of incidence of choline-deficiency depends upon different factors i.e. choline requirements in premenopausal women are decreased since the endogenous biosynthesis (de novo) of choline in the liver is estrogen-sensitive (11). In women, a single nucleotide polymorphism that has been identified in the PEMT gene is responsible for estrogen-induction of de novo biosynthesis of choline (11,12), thus it seems that choline-deficiency could occur as a genetically promoted disorder.
Choline-deficiency
The role of choline is crucial and its deficiency is considered an unhealthy state that leads to body organ dysfunction both in humans and animals (13). Insufficient dietary intake can be observed in about two weeks (14-16). Although choline is ubiquitous in different food items—a fact that makes choline-deficiency rare—choline-deficiency can be seen in physiological (e.g., intensive exercise, pregnancy and lactation) and pathological states (e.g., alcoholism and malnutrition) (17,18). Dietary lecithin (phosphatidylcholine) is considered the common source of choline; most of the choline mass is stored in phospholipid-bound form. Plasma free-choline depletion has been associated with the development of hepatic disorder (19). A very susceptible group to choline deficiency are patients on parenteral nutrition (PN); these patients have limited absorption capabilities (20), a fact that further deteriorates liver structure and function (19,21). It is noteworthy that in addition to hepatic metabolism, some of the dietary choline is degraded by intestinal flora before absorption (7,22). Despite the fact that PN contains the choline precursor “Methionine”, the plasma-free choline concentration has been found significantly lower compared to normal levels in patients on PN in both adults and older children and it has been associated with increased liver enzymes (20,23-25).
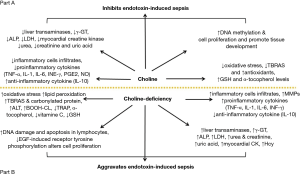
There are many studies that have uncovered several pathological conditions under the impact of choline-deficiency such as steatohepatitits, cirrhosis, hepatic cell degeneration and hepatocellular carcinoma (17,26-28), disruption of normal glucose metabolism and induction of insulin resistance (18,29), impairment in cardiac function with structural changes (30), renal tubular and cortical necrosis (31,32), ocular haemorrhagic lesions and disruption of brain development and cognitive function (33,34). In addition, choline-deficiency induces metabolic derangement leading to organ damage through the triggering of different pathways, including tumor necrosis factor alpha (TNF-α), hyperhomocysteinemia and lipid peroxidation (20). Ossani et al. (32) and Repetto et al. (35) reported that dietary choline-deficiency induces oxidative damage in the liver, kidney, heart and brain with an increment in lipid peroxidation (in rats there is an increase in plasma levels of oxidative agents and a decrease in plasma levels of antioxidants). Repetto et al. (35) found a 33% decrease in the total reactive antioxidant potential (TRAP) plasma level due to a decrease in the intracellular glutathione (GSH) and a decrease in tissue lipid soluble α-tocopherol, and a 5-fold increase in thiobarbituric acid-reactive substances (TBARS) plasma level due to stimulation of lipid peroxidation. These changes usually precede the appearance of irreversible histopathological damage of the organs. In vitro studies revealed that hepatocytes that have grown in choline-deficient media produced increased reactive oxygen species (ROS) from mitochondria in comparison to the ones grown in choline-rich media (36-41). In rats, dietary choline-deficiency caused an accumulation of lipid peroxides which led to further DNA damage in hepatocytes (42) and lymphocytes (43).
Choline-deficiency has been shown to decrease the activities of lysozyme and acid phosphatase, contents of complement 3 and immunoglobins (IgM), and to downregulate the mRNA levels of antimicrobial peptides, liver-expressed antimicrobial peptides (LEAP-2A, LEAP-2B), defensin-3 and hepcidin in the intestinal segments of juvenile Jian carp; furthermore choline-deficiency impaires the intestinal antimicrobial defense of juvenile Jian carp (44). Notably, that choline acts also as an endogenous cholinergic agonist (45); since experiments in rats have shown that cholinergic agonists induce an increase in the secretion of lysozyme and defensin in the intestine (46), the impaired intestinal antimicrobial defense caused by choline-deficiency might be partially related to the decreased cholinergic agonist levels (44). In addition, a study by Wu et al. (44) found that choline-deficiency in fish suppressed intestinal antimicrobial defense by decreasing antimicrobial component levels and induced intestinal inflammation via upregulation of pro-inflammatory cytokines’ expression and downregulation of anti-inflammatory cytokines’ expression. Conversely, Wu et al. (47) have shown that diet rich in choline enhances serum lysozyme activity and complement 3 content, decreases pro-inflammatory cytokines interleukin 1 beta (IL-1β) and TNF-α mRNA levels while increases anti-inflammatory cytokines IL-10 mRNA levels in the main immune organs of juvenile Jian carp. The same researchers revealed that choline-deficiency upregulated the mRNA levels of nuclear factor kappa of activated B cell (NF-κB) and increased signal transducer and activator of transcription proteins (STAT) signaling pathways whereas downregulated mRNA levels of cellular protein inhibitor of kappa B (IκB) in the intestine of fish (44). In mammals, NF-κB is one of the critical signaling molecules for regulating transcription of cytokines (48) and its overactivation aggravates inflammatory reactions in rats (49). In fish, the upregulated mRNA level of proinflammatory cytokine TNF-α and the downregulated mRNA level of anti-inflammatory cytokines IL-10 and transforming growth factor-beta 2 (TGF-β2) could be related to the changes of signaling molecules NF-κB and IκB in the intestine caused by the choline-deficiency (44). Furthermore, choline-deficiency upregulated the mRNA level of Toll-like receptor 4 and Myeloid differentiation primary response 88 (MyD88) in the intestine of the fish (44,50).
Choline-deficiency promotes cellular apoptosis due to defective DNA repair (51-53). Previous reports have shown that consumption of a choline-deficient diet leads to reversible hepatocellular modifications characterized by hepatosteatosis, liver and muscle damage and increased lymphocytes apoptosis (53-56); in addition, da Costa et al. (54); James et al. (57) and Shin et al. (58) reported hepatocyte and myocyte death when cultured in a choline-deficient media that may justify the elevation of serum liver enzymes and creatine phosphokinase in human blood when humans are subjected to choline-deficiency.
The above mentioned facts and findings motivated the scientists and researchers to continue working on the disruption of crucial physiological processes as well as on the deterioration of many pathological conditions under the impact of choline-deficiency.
This review focuses on (I) the role of choline as an essential substance in the control and modulation of different immunological and inflammatory pathways in multiple models of sepsis (animal and human); (II) the consequences of choline-deficiency on the immunological and inflammatory response, and ultimately; (III) the importance of the nutritional status in septic patients.
Choline and sepsis
Sepsis is a life-threatening condition that arises when the body’s immune response is provoked against an infection. In modern medicine, sepsis remains as a critical clinical condition associated with high rates of morbidity and mortality (59). A major challenge in intensive care medicine is the treatment of a serious infection related to multiple organ dysfunction, generally termed as sepsis, severe sepsis or septic shock (60), which is considered as a critical and costly condition in the intensive care units worldwide. Gram-negative bacteria are the most common cause of septic shock. The toxic effects of gram-negative bacteria are due to a non-secreted, heat-stable endotoxin called lipopolysaccharide (LPS) (61,62).
A series of inflammatory reactions called systemic inflammatory response syndrome are triggered by LPS. TNF-α and ILs secreted by LPS-activated cells into the systemic circulation cause a stimulation of the hepatic cells to release acute phase proteins such as C-reactive protein (CRP) for immunological regulation (45,63); an immunological imbalance is associated with a deteriorated outcome of septic patients (64). Matrix metalloproteinases (MMPs) released into the circulation from damaged vascular endothelium might also have a role in the pathophysiology of sepsis (65). MMPs production is up-regulated by pro-inflammatory cytokines (TNF-α, IL-1 and IL-17), as well as by acute phase proteins (serum amyloid A), with counter-regulatory inhibition by IL-4 and IL-13 (66,67). Some MMPs regulate cytokine and other inflammatory molecular responses after the initiation of sepsis by activating protease-activated receptor-1 Jeny(67-69); the increased MMP/TIMP ratio (TIMP is tissue inhibitor metalloproteinases) seems to be more related to a tissue response to LPS linked injury rather, than to their involvement in the acute phase reaction (67).
Findings of Kocaturk et al. in 2016 (70) showed that choline treatment suppressed the increased MMPs and TIMPs serum concentrations in experimentally-induced sepsis in male and female mongrel dogs (0.2 mg/kg intravenous LPS-Escherichia coli); the increase was related to the acute phase response and organ damage, while choline prevented the reduced of Igs (IgM, IgG) concentration induced by endotoxinaemia (70).
Many researchers have explored the significant correlation between choline and immunity in human and animals (44,45,47,71-73). Nolan and Vilayat in 1968 (74) reported that the hepatic injury and mortality due to endotoxinaemic shock induced by intraperitoneal injection of LPS-Escherichia coli, was significantly increased in adult female Holtzman rats fed on a choline-deficient diet; on the contrary, Rivera et al. (75) showed that a choline-rich diet protects the liver and improves survival rates in endotoxinaemic shock induced by intravenous injection of LPS-Escherichia coli in female Sprague-Dawley rats. With regards to sepsis and especially conditions that simulate sepsis i.e., a stressful surgery, it has been noticed that serum-free choline decreased during and after elective abdominal surgery, total abdominal hysterectomy, vaginal or cesarean childbirth, brain tumor resection and traumatic brain injury (76,77). Laboratory studies have shown that serum-free and phospholipid-bound choline concentrations decline in response to surgical and traumatic injuries in humans (76,78,79) and in dogs (77). Moreover, a stressful condition can induce a variety of metabolic and neuroendocrine changes (80-83) including the increase of cortisone, prolactin, adrenocorticotrophic hormone and β-endophrine (80,83-85). In 2002, Ilcol et al. (78) found that serum choline levels were inversely correlated with the levels of stress hormones. Furthermore, under the insult of choline-deficiency, the endogenous biosynthesis (de novo) of phosphatidylcholine is promoted to compensate the demands in choline leading to increased homocysteine (86); the latter is involved in the regulation of cytokines and inflammation (87,88). In humans increased dietary choline leads to decrease in the homocysteine levels (89).
Furthermore, choline treatment (I) improved the hematological and serum biochemical findings of endotoxin-induced sepsis [0.02 or 1 mg/kg intravenous LPS-Escherichia coli in a saline solution] in adult mongrel dogs via activation of alpha7 nicotinic acetylcholine receptor (α7nAChR) (90,91); (II) attenuated the endotoxin-induced decrease in serum activity of butyrylcholinesterase and paraoxonase 1, and to a lesser extent the increases of CRP, haptoglubin and ceruloplasmin during experimentally induced sepsis in adult male and female mongrel dogs (92); (III) attenuated the increase of serum acute phase proteins (93) which are involved in the cholinergic anti-inflammatory pathway (90,91) and (IV) attenuated and even in some cases suppressed the increased MMPs and TIMPs but did not affect MMP-2 in response to a single dose of LPS-induced endotoxinaemia (70). The latter effect could be ascribed to the contribution of choline in maintaining endothelial integrity, membrane phospholipids’ structural integrity (94), and down-regulation of TNF-α expression (91). TNF-α has been reported to up- or down-regulate the expression of MMP-9, MMP-13, and MMP-14 (95) while nicotine treatment was reported to decreases expression of MMP-14 (96).
Administration of choline inhibited the harmful effects evoked by endotoxin on the vascular bed damage and leakage and protected immunoglobulin responses to LPS in lymphocytes (70,90,97) and other immune and non-immune cytokine producing cells (90). Furthermore, intracerebroventricular administration of the choline metabolite LysoPC reversed the hypotension and protected against lethality induced by endotoxin (98). Thus, it has also been suggested that choline-containing phospholipids, like lysoPC (98) and phospholipids have therapeutic effects (98,99) and improve survival in experimental models of sepsis induced by cecal ligation and puncture or intraperitoneal injection of Escherichia coli in mice (98) as well as in Yorkshire pigs (99).
Ilcol et al. (90) showed that experimental endotoxin-induced sepsis in dogs altered circulating choline status in a dose and time dependent manner; choline levels were in relation to serum cortisol and markers for tissue injury and/or organ dysfunction (90). Endotoxinaemia is accompanied by liver and kidney dysfunction; liver and/or kidneys excision in dogs slows clearance of free choline from circulation (100) while renal failure results in elevated levels of serum-free choline in human (101-104). In adult mongrel dogs injected with sublethal dose of endotoxin (1 mg/kg), serum levels of aspartate aminotransferase, alanine transaminase, alkaline phosphatase, gamma-glutamyltransferase, lactate dehydrogenase, creatine kinase, creatine kinase-MB, urea, creatinine, and uric acid decreased by choline administration (90) indicating that choline protects, at least in part, liver, renal, skeletal, and cardiac muscle injuries. Choline’s ability to attenuate endotoxin-induced elevations of biochemical markers for tissue injury and/or organ dysfunction was much higher at a high-dose (1 mg/kg) rather than a low dose (0.02 mg/kg) in endotoxin-treated dogs; according to Ilcol et al. (105) the mechanism of choline protection against experimentally induced endotoxin, could be attributed to increased availability of free choline and the consequent increase of central (1,106) and/or peripheral cholinergic neurotransmission (105); the increased cholinergic neurotransmission in the peripheral parasympathetic system could lead to an activation of vagal anti-inflammatory systems (105,107,108) and subsequent inhibition of endotoxin-induced toxic mediators from endotoxin-sensitive cells (Figure 1).
Furthermore, choline attenuates the elevation in serum TNF-α in response to 1 mg/kg dose of endotoxin, while a choline-rich diet decreases serum TNF-α by inhibiting its release from the Kupffer cells (75). Increased availability of free choline could increase membrane phospholipid synthesis (1,106,115) and/or decrease membrane breakdown (116) and diminish the vulnerability of tissues to elevated toxic mediators during endotoxinaemia. In dogs, circulating choline status is altered during experimental endotoxinaemia (72). In asthmatic patients, choline treatment decreases TNF-α, IL-4 an IL-5 release from mononuclear cells (73); in addition to that, ACh, a major choline metabolite, inhibits the production of TNF-α and IL-1 from human macrophage (117) and lymphocytes (118) and mouse microglia (119) via α7nAChR (Figure 1).
In non-terrestrial animals, like juvenile Jian carp (Cyprinus carpio var. Jian), it has been shown that when dietary choline was increased up to a certain level, the inflammation induced against Aeromonas hydrophilia challenge (intrapertoneal injection with Aeromonas hydophilia as a semilethal dose of endotoxin) was attenuated via decrease of TNF-α, IL-1β, and TGF-β2 mRNA relative expression in the immune organs (47). In line with TNF-α expression in liver (120), TGFβ1expression in rat hippocampus decreased after choline supplementation (121). Furthemore, mammalian target of rapamycin (mTOR) signaling pathways have been found to be involved in the function of the immune system (122), i.e., in mice, inhibition of mTOR reduced the release of TNF-α, IL-6 and IL-10 from activated macrophages (123). In addition, mTOR activates interferon regulatory factor-5 and -7 which are considered the principal transcription factors for pro-inflammatory cytokine genes in activated HEK293T cell-line (123). In female mice macrophage, phosphatidic acid, the hydrolysis product of PtdCho, enhanced the production of TNF-α, IL-1β, IL-6, nitric oxide and prostaglandin E2 by regulating the activity of mTOR-p70S6K1 (124). Wu et al. (125) found that dietary choline regulated the relative gene expressions of mTOR and the eukaryotic initiation factor 4E-binding protein-2 (4E-BP2) in muscle, hepatopancreas and intestine of juvenile Jian carp; thus, choline may also affect cytokines’ release through modulation of mTOR pathway (47).
Wu et al. (47,125) showed that dietary choline could enhance fish disease resistance and improve the survival of fish when subjected to Aeromonas hydrophilia challenge suggesting that dietary choline regulates the inflammation and enhances non-specific and specific immunity through serum activities of lysozyme and lysosome acid phosphate, hemagglutination titer, content of the complement 3 and 4, and leucocytes phagocytic activity of fish after challenge. In addition, according to Parrish et al. (45) endogenous choline may act on the α7nAChR and play an important role in regulating innate immune responses to maintain homeostasis. On the contrary, choline-deficiency decreased the survival in cobia fish (126) and hydro tilapia (127) and resulted in severe destruction of the mid-gut gland epithelial cells of juvenile shrimp (128).
In mice, both T and B lymphocytes express multiple muscarinic and nicotinic acetylcholine receptors (mAChRs and nAChRs, respectively); ACh can bind to mAChRs and nAChRs on T and B cells leading to modulation of their function (129); in a rat cultured spleen cell, ACh enhanced the Con A-induced T-cell proliferation (130). ACh also promoted anti-inflammatory response by mediating vagus nerve-based cholinergic anti-inflammatory response (48). Despite the fact that choline acts as a selective α7nAChR agonist (45), it failed to inhibit the systemic level of TNF-α in knockout mice during endotoxinaemia (45), indicating that choline may partially mediate cytokines’ expression via α7nAChR signaling. Therefore, the immunoregulatory effect of choline on the immune system could ascribe its effect on the cholinergic system (Figure 1).
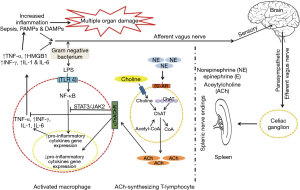
NF-κB is a crucial protein complex for DNA transcription and cell survival, it regulates gene expression of the inflammatory cytokines in macrophages, monocytes and endothelial cells (48). Choline markedly decreased TNF-α level associated with suppressed activation of NF-κB in endotoxin-stimulated RAW-264.7 mouse macrophage-like cell (45). Moreover, NF-κB is an important transcriptional activator that regulates RNAs transcription (48). In fact, choline-deficiency impairs global DNA methylation (131) (Figure 2).
Furthermore, in juvenile Jian carp fish, choline-deficiency was associated with a decrease in red blood cells (RBC) and white blood cells (WBC) while RBC count increased by increasing the dietary choline levels to a certain level (47). This fact indicates that choline contributes to innate immunity (45) since low RBC and WBC levels decrease the immunity and increase the susceptibility to diseases (47,132).
Taking into consideration that the development and the growth of tissues and organs depend on cell proliferation (133) and the latter depends on the structural integrity of cells (134), it seems that choline is a major contributor in these vital processes through its essential role to maintain the structural integrity of the cell biological membranes (135) and DNA biosynthesis and repair (136).
Kortstee in 1970 (137) reported that several aerobic microorganisms can decompose choline and grow with choline as the sole C- and N-source in vitro; meanwhile, the gut microflora can metabolize choline to trimethylamine (138), suggesting that choline may play an important role in controlling intestinal microflora. On the other hand, dietary choline administration induced a significant increase in intestinal lactobacillus microflora count, while intestinal Escherichia coli and Aeromonas hydrophila counts were the lowest (47).
Conclusions
This review draws a picture of the role of choline in the manipulation and modification of the different pathological responses under the impact of septic insult that has been triggered in different models. The protective and supportive role of choline in a war against sepsis and inflammatory diseases has been predicted through assessment of different findings in different studies. However, the understanding of the molecular pathophysiology of sepsis and of the role of choline in it, is far from complete.
Acknowledgments
This study was supported by Medical School of National and Kapodistrian University of Athens.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science 1983;221:614-20. [Crossref] [PubMed]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr 1994;14:269-96. [Crossref] [PubMed]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229-50. [Crossref] [PubMed]
- Food and Nutrition Board (Institute of Medicine). Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Pantothenic Acid, Biotin and Choline. National Academy Press, Washington, D.C, 1998.
- Li Z, Vance D. Phosphatidylcholine and choline homeostasis. J Lipid Res 2008;49:1187-94. [Crossref] [PubMed]
- Cheng WL, Holmes-McNary MQ, Mar MH, et al. Bioavailability of choline and choline esters from milk in rat pups. J Nutr Biochem 1996;7:457-64. [Crossref]
- Le Kim D, Betzing H. Intestinal absorption of polyunsaturated phosphatidyl-choline in the rat. Hoppe Seylers Z. Physiol Chem 1976;357:1321-31. [Crossref] [PubMed]
- Zeisel SH, Mar MH, Howe JC, et al. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302-7. [Crossref] [PubMed]
- Cui Z, Vance DE. Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. J Biol Chem 1996;271:2839-43. [Crossref] [PubMed]
- da Costa KA, Corbin KD, Niculescu MD, et al. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J 2014;28:2970-8. [Crossref] [PubMed]
- Resseguie ME, da Costa KA, Galanko JA, et al. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J Biol Chem 2011;286:1649-58. [Crossref] [PubMed]
- Ganz AB, Klat KC, Caudill MA. Common Genetic Variants Alter Metabolism and Influence Dietary Choline Requirements. Nutrients 2017;9. [Crossref] [PubMed]
- Sanders LM, Zeisel SH. Choline: Dietary Requirements and Role in Brain Development. Nutr Today 2007;42:181-6. [Crossref] [PubMed]
- Pomfret EA, da Costa KA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon rat liver. J Nutr Biochem 1990;1:533-41. [Crossref] [PubMed]
- Zeisel SH, Zola T, da Costa KA, et al. Effect of choline deficiency on S-adenosyl methionine and methionine concentrations in rat liver. Biochem J 1989;259:725-9. [Crossref] [PubMed]
- Zeisel SH, da Costa KA, Fraklin PD, et al. Choline: an essential nutrient for humans. FASEB J 1991;5:2093-8. [Crossref] [PubMed]
- Lombardi B, Pani P, Schlunk FF. Choline- deficiency fatty liver: impaired release of hepatic triglycerides. J Lipid Res 1968;9:437-46. [PubMed]
- Veteläinen R, van Vliet A, van Gulik TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methionine- choline deficient diets in a rat model. J Gastroenterol Hepatol 2007;22:1526-33. [Crossref] [PubMed]
- Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev 2009;67:615-23. [Crossref] [PubMed]
- Buchman AL. The addition of choline to parenteral nutrition. Gastroenterology 2009;137:S119-28. [Crossref] [PubMed]
- Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999;117:1043-50. [Crossref] [PubMed]
- Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamine from ingested choline and lecithin. J Pharmacol Exp Ther 1983;225:320-4. [PubMed]
- Misra S, Ahn C, Ament ME, et al. Plasma choline concentrations in children requiring long-term home parenteral nutrition: a case control study. JPEN J Parenter Enteral Nutr 1999;23:305-8. [Crossref] [PubMed]
- Vinton NE, Laidlaw SA, Ament ME, et al. Taurine concentrations in plasma and blood cells in patients undergoing long-term parenteral nutrition. Am J Clin Nutr 1986;44:398-404. [Crossref] [PubMed]
- Dahlström KA, Ament ME, Laidlaw AW, et al. Plasma amino acid concentrations in children receiving long-term parenteral nutrition. J Pediatr Gastroenterol Nutr 1988;7:748-54. [Crossref] [PubMed]
- Chandar N, Lombardi B. Liver cell proliferation and incidence of hepatocellular carcinomas in rats fed consecutively a choline-devoid and a choline-supplemented diet. Carcinogenesis 1988;9:259-63. [Crossref] [PubMed]
- da Costa KA, Cochary EF, Blusztajn JK, et al. Accumulation of 1,2-sn-diradylglycerol with increased membrane associated protein kinase C may be the mechanism for spontaneous hepatocarcinogenesis in choline-deficient rats. J Biol Chem. 1993;268:2100-5. [PubMed]
- Al-Humadi H, Theocharis S, Dontas I, et al. Hepatic injury due to combined choline-deprivation and thioacetamide administration: an experimental approach to liver diseases. Dig Dis Sci 2012;57:3168-77. [Crossref] [PubMed]
- Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 2012;28:159-65. [Crossref] [PubMed]
- Strilakou AA, Lazaris AC, Perelas AI, et al. Heart dysfunction induced by choline-deficiency in adult rats: the protective role of L-carnitine. Eur J Pharmacol 2013;709:20-7. [Crossref] [PubMed]
- Kumagai H, Katoh S, Hirosawa K. Renal tubulointerstitial injury in weanling rats with hyperhomocysteinemia. Kidney Int 2002;62:1219-28. [Crossref] [PubMed]
- Ossani G, Dalghi M, Repetto M. Oxidative damage and lipid peroxidation in the kidney of choline deficient rats. Front. Biosci 2007;12:1174-83. [Crossref] [PubMed]
- Ossani GP, Pelayes D, Diaz ML, et al. Ocular lesions and experimental choline deficiency. Medicina (B Aires) 2006;66:415-20. [PubMed]
- Mellott TJ, Follettie MT, Diesl V, et al. Prenatal choline availability modulates hippocampal and cerebral cortical gene expression. FASEB J 2007;21:1311-23. [Crossref] [PubMed]
- Repetto MG, Ossani G, Monserrat AJ, et al. Oxidative damage: the biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp Mol Pathol 2010;88:143-9. [Crossref] [PubMed]
- Rushmore TH, Lim YP, Farber E, et al. Rapid lipid peroxidation in the nuclear fraction of rat liver induced by a diet deficient in choline and methionine. Cancer Lett 1984;24:251-5. [Crossref] [PubMed]
- Banni S, Corongiu FP, Dessi MA, et al. Free radicals and lipid peroxidation in liver of rats kept on a diet devoid of choline. Free Radic Res Commun 1989;7:233-40. [Crossref] [PubMed]
- Yoshida LS, Miyazawa T, Fujimoto K, et al. Liver phosphatidylcholine hydroperoxidation provoked by ethionine-containing cholinedeficient diet in mice. Lipids 1990;25:565-9. [Crossref] [PubMed]
- Ghoshal AK, Farber E. Liver biochemical pathology of choline deficiency and of methyl group deficiency: a new orientation and assessment. Histol Histopathol 1995;10:457-62. [PubMed]
- Vrablic AS, Albright CD, Craciunescu CN, et al. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methylrac- glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J 2001;15:1739-44. [Crossref] [PubMed]
- Albright CD, Salganik RI, Craciunescu CN, et al. Mitochondrial and microsomal derived reactive oxygen species mediate apoptosis induced by transforming growth factor-beta1 in immortalized rat hepatocytes. J Cell Biochem 2003;89:254-61. [Crossref] [PubMed]
- Pogribny IP, Basnakian AG, Miller BJ, et al. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res 1995;55:1894-901. [PubMed]
- James SJ, Yin L. Diet-induced DNA damage and altered nucleotide metabolism in lymphocytes from methyl-donor-deficient rats. Carcinogenesis 1989;10:1209-14. [Crossref] [PubMed]
- Wu P, Jiang WD, Jiang J, et al. Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juvenile Jian carp. Fish Shellfish Immunol 2016;58:462-73. [Crossref] [PubMed]
- Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, et al. Modulation of TNF release by choline requires a7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med 2008;14:567-74. [Crossref] [PubMed]
- Qu XD, Lloyd KC, Walsh JH, et al. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal paneth cells. Infect Immun 1996;64:5161-5. [PubMed]
- Wu P, Jiang J, Liu Y, et al. Dietary choline modulates immune responses, and gene expressions of TOR and eIF4E-binding protein2 in immune organs of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 2013;35:697-706. [Crossref] [PubMed]
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 2007;151:915-29. [Crossref] [PubMed]
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013;12:86. [Crossref] [PubMed]
- Zhu LY, Nie L, Zhu G, et al. Advances in research of fish immune relevant genes: a comparative overview of innate and adaptive immunity in teleosts. Dev Comp Immunol 2013;39:39-62. [Crossref] [PubMed]
- Zeisel SH, da Costa KA, Albright CD, et al. Choline and hepatocarcinogenesis in the rat. Adv Exp Med Biol 1995;375:65-74. [Crossref] [PubMed]
- Albright CD, da Costa KA, Craciunescu CN, et al. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol Biochem 2005;15:59-68. [Crossref] [PubMed]
- da Costa KA, Niculescu MD, Craciunescu CN, et al. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr 2006;84:88-94. [Crossref] [PubMed]
- da Costa KA, Gaffney CE, Fischer LM, et al. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr 2005;81:440-4. [Crossref] [PubMed]
- Kohlmeier M, da Costa KA, Fischer LM, et al. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A 2005;102:16025-30. [Crossref] [PubMed]
- da Costa KA, Kozyreva OG, Song J, et al. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J 2006;20:1336-44. [Crossref] [PubMed]
- James SJ, Miller BJ, Basnakian AG, et al. Apoptosis and proliferation under conditions of deoxynucleotide pool imbalance in liver of folate/methyl deficient rats. Carcinogenesis 1997;18:287-93. [Crossref] [PubMed]
- Shin OH, Mar MH, Albright CD, et al. Methyl-group donors cannot prevent apoptotic death of rat hepatocytes induced by choline deficiency. J Cell Biochem 1997;64:196-208. [Crossref] [PubMed]
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303-10. [Crossref] [PubMed]
- László I, Trásy D, Molnár Z, et al. Sepsis: From Pathophysiology to Individualized Patient Care. J Immunol Res 2015;2015:510436. [Crossref] [PubMed]
- Erridge C, Bennett-Guerrero E, Poxton IR. Structure and function of lipopolysaccharides. Microbes Infect 2002;4:837-51. [Crossref] [PubMed]
- Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev 2003;16:379-414. [Crossref] [PubMed]
- Karlsson I, Wernersson S, Ambrosen A, et al. Increased concentrations of C-reactive protein but not high-mobility group box 1 in dogs with naturally occurring sepsis. Vet Immunol Immunopathol 2013;156:64-72. [Crossref] [PubMed]
- Bermejo-Martin JF, Giamarellos-Bourboulis EJ. Endogenous immunoglobulins and sepsis: New perspectives for guiding replacement therapies. Int J Antimicrob Agents 2015;46 Suppl 1:S25-28. [Crossref] [PubMed]
- Lauhio A, Hästbacka J, Pettilä V, et al. Serum MMP-8, -9 and TIMP-1 in sepsis: high serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol Res 2011;64:590-4. [Crossref] [PubMed]
- Ally MM, Hodkinson B, Meyer PW, et al. Serum matrix metalloproteinase-3 in comparison with acute phase proteins as a marker of disease activity and radiographic damage in early rheumatoid arthritis. Mediators Inflamm 2013;2013:183653. [Crossref] [PubMed]
- Martin G, Asensi V, Montes AH, et al. Role of plasma matrix-metalloproteases (MMPs) and their polymorphisms (SNPs) in sepsis development and outcome in ICU patients. Sci Rep 2014;4:5002. [Crossref] [PubMed]
- Tressel SL, Kaneider NC, Kasuda S, et al. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol Med 2011;3:370-84. [Crossref] [PubMed]
- Wang M, Zhang Q, Zhao X, et al. Diagnostic and prognostic value of neutrophil gelatinase-associated lipocalin, matrix metalloproteinase-9, and tissue inhibitor of matrix metalloproteinases-1 for sepsis in the Emergency Department: an observational study. Crit Care 2014;18:634. [Crossref] [PubMed]
- Kocaturk M, Eralp-Inan O, Tvarijonaviciute A, et al. Effects of choline treatment in concentrations of serum matrix metalloproteinases (MMPs), MMP tissue inhibitors (TIMPs) and immunoglobulins in an experimental model of canine sepsis. Vet Immunol Immunopathol 2016;180:9-14. [Crossref] [PubMed]
- Eastin CE, McClain CJ, Lee EY, et al. Choline deficiency augments and antibody to tumor necrosis factor- attenuates endotoxin induced hepatic injury. Alcohol Clin Exp Res 1997;21:1037-41. [Crossref] [PubMed]
- Yilmaz Z, Ilcol YO, Torun S, et al. Intravenous administration ofcholine or cdp-choline improves platelet count and platelet closure times inendotoxin-treated dogs. Shock 2006;25:73-9. [Crossref] [PubMed]
- Mehta AK, Singh B, Arora N, et al. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology 2010;215:527-34. [Crossref] [PubMed]
- Nolan JP, Vilayat M. Endotoxin and the liver. 1. Toxicity in rats and choline deficient fatty livers. Proc Soc Exp Biol Med 1968;129:29-31. [Crossref] [PubMed]
- Rivera CA, Wheeler MD, Enomoto N, et al. A choline rich diet improves survival in a rat model of endotoxin shock. Am J Physiol 1998;275:G862-67. [PubMed]
- Ulus IH, Ozyurt G, Korfali E. Decreased serum choline concentrations in humans after surgery, childbirth, and traumatic head injury. Neurochem Res 1998;23:727-32. [Crossref] [PubMed]
- Ilcol YO, Uncu G, Goren S, et al. Declines in serum free and phospholipid-bound choline concentrations in humans after three different types of major surgery. Clin Chem Lab Med 2004;42:1390-5. [Crossref] [PubMed]
- Ozarda Ilçöl Y, Ozyurt G, Kilicturgay S, et al. The decline in serum choline concentration in humans during and after surgery is associated with the elevation of cortisol, adrenocorticotropic hormone, prolactin and beta-endorphin concentrations. Neurosci Lett 2002;324:41-4. [Crossref] [PubMed]
- Ozarda Ilcol Y, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch Physiol Biochem 2002;110:393-9. [Crossref] [PubMed]
- Dubois M, Pickar D, Cohen MR, et al. Surgical stress in humans is accompanied by an increase in plasma beta-endorphin immunoreactivity. Life Sci 1981;29:1249-54. [Crossref] [PubMed]
- Haxholdt OS, Kehlet H, Dyrberg V. Effect of fentanyl on the cortisol and hyperglycemic response to abdominal surgery. Acta Anaesthesiol. Scand 1981;25:434-6. [Crossref] [PubMed]
- Deuss U, Dietrich J, Kaulen D, et al. The stress response to laparoscopic cholecystectomy: investigation of endocrine parameters Endoscopy 1994;26:235-8. [Crossref] [PubMed]
- Kehlet H. Surgical stress response: does endoscopic surgery confer an advantage? World J Surg 1999;23:801-7. [Crossref] [PubMed]
- Noel GL, Suh HK, Stone JG, et al. Human prolactin and growth hormone release during surgery and other conditions of stress. J Clin Endocrinol Metab 1972;35:840-51. [Crossref] [PubMed]
- Lacoumenta S, Yeo TH, Burrin JM, et al. Fentanyl and the b-endorphin, ACTH and glucoregulatory hormonal response to surgery. Br J Anaesth 1987;59:713-20. [Crossref] [PubMed]
- Zeisel SH. Choline homocysteine and pregnancy. Am J Clin Nutr 2005;82:719-20. [Crossref] [PubMed]
- Shai I, Stampfer MJ, Ma J, et al. Homocysteine as a risk factor for coronary heart diseases and its association with inflammatory biomarkers, lipids and dietary factors. Atherosclerosis 2004;177:375-81. [Crossref] [PubMed]
- Su SJ, Huang LW, Pai LS, et al. Homocysteine at pathophysiologic concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrition 2005;21:994-1002. [Crossref] [PubMed]
- Cho E, Zeisel SH, Jacques P, et al. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr 2006;83:905-11. [Crossref] [PubMed]
- Ilcol YO, Yilmaz Z, Ulus IH. Endotoxin alters serum-free choline andphospholipid-bound choline concentrations: and choline administrationattenuates endotoxin-induced organ injury in dogs. Shock 2005;24:288-93. [Crossref] [PubMed]
- Pavlov VA, Wang H, Czura CJ, et al. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 2003;9:125-34. [Crossref] [PubMed]
- Tvarijonaviciute A, Kocaturk M, Cansev M, et al. Serum butyrylcholinesterase and paraoxonase 1 in a canine model ofendotoxemia: effects of choline administration. Res Vet Sci 2012;93:668-74. [Crossref] [PubMed]
- Tvarijonaviciute A, Eralp O, Kocaturk M. Adiponectinand IGF-1 are negative acute phase proteins in a dog model of acute endotoxaemia. Vet Immunol Immunopathol 2011;140:147-51. [Crossref] [PubMed]
- Yilmaz Z, Ozarda Y, Cansev M, et al. Choline or CDP-choline attenuates coagulation abnormalities and prevents the development of acute disseminated intravascular coagulation in dogs during endotoxemia. Blood Coagul Fibrinolysis 2010;21:339-48. [Crossref] [PubMed]
- Chen Y, Guo Q, Pan X, et al. Smoking and impaired bone healing: will activation of cholinergic anti-inflammatory pathway be the bridge? Int Orthop 2011;35:1267-70. [Crossref] [PubMed]
- Leib C, Göser S, Lüthje D, et al. Role of the cholinergic antiinflammatory pathway in murine autoimmune myocarditis. Circ Res 2011;109:130-40. [Crossref] [PubMed]
- Ilcol YO, Yilmaz Z, Cansev M, et al. Choline or CDP-choline altersserum lipid responses to endotoxin in dogs and rats: involvement of theperipheral nicotinic acetylcholine receptors. Shock 2009;32:286-94. [Crossref] [PubMed]
- Yan JJ, Jung JS, Lee JE, et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med 2004;10:161-7. [Crossref] [PubMed]
- Goldfarb RD, Parker TS, Levine DM, et al. Protein-free phospholipid emulsion treatment improved cardiopulmonary function and survival in porcine sepsis. Am J Physiol Regul Integr Comp Physiol 2003;284:R550-7. [Crossref] [PubMed]
- Bligh J. The role of the liver and the kidneys in the maintenance of the level of free choline in plasma. J Physiol 1953;120:53-62. [Crossref] [PubMed]
- Rennick B, Acara M, Hysert P, et al. Choline loss during hemodialysis: homeostatic control of plasma concentrations. Kidney Int 1976;10:329-35. [Crossref] [PubMed]
- Buchman AL, Jenden D, Suki WN, et al. Changes in plasma free choline and phospholipid-bound choline in chronic hemodialysis patients. J Ren Nutr 2000;10:133-8. [Crossref] [PubMed]
- Ilcol YO, Dilek K, Yurtkuran M, et al. Changes of plasma free choline and choline containing compounds’ concentrations and choline loss during hemodialysis in ESRD patients. Clin Biochem 2002;35:233-9. [Crossref] [PubMed]
- Ilcol YO, Donmez O, Yavuz M, et al. Free choline and phospholipid-bound choline concentrations in serum and dialysate during peritoneal dialysis in children and adults. Clin Biochem 2002;35:307-13. [Crossref] [PubMed]
- Ilcol YO, Gurun MS, Taga Y, et al. Choline increases serum insulin in rat when injected intraperitoneally and augments basal and stimulated acetylcholine release from the rat minced pancreas in vitro. Eur J Biochem 2003;270:991-9. [Crossref] [PubMed]
- Wurtman RJ. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci 1992;15:117-22. [Crossref] [PubMed]
- Downing JE, Miyan JA. Neurol immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today 2000;21:281-9. [Crossref] [PubMed]
- Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011;334:98-101. [Crossref] [PubMed]
- Delgado-Vélez M, Lasalde-Dominicci JA. The Cholinergic Anti-Inflammatory Response and the Role of Macrophages in HIV-Induced Inflammation. Int J Mol Sci 2018;19:1473. [Crossref] [PubMed]
- Wu H, Li L, Su X. Vagus nerve through α7 nAChR modulates lung infection and inflammation: models, cells, and signals. Biomed Res Int 2014;2014:283525. [Crossref] [PubMed]
- Hosoi T, Okuma Y, Matsuda T, et al. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci 2005;120:104-7. [Crossref] [PubMed]
- Liu L, Lu Y, Bi X, et al. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci Rep 2017;7:42553. [Crossref] [PubMed]
- Lips KS, Lührmann A, Tschernig T, et al. Down-regulation of the non-neuronal acetylcholine synthesis and release machinery in acute allergic airway inflammation of rat and mouse. Life Sci 2007;80:2263-9. [Crossref] [PubMed]
- de Jonge WJ, van der Zanden EP. The FO. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 2005;6:844-51. [Crossref] [PubMed]
- Stoll AL, Renshaw PF, De Micheli E, et al. Choline ingestion increases the resonance of choline-containing compounds in human brain: an in vivo proton magnetic resonance study. Biol Psychiatry 1995;37:170-4. [Crossref] [PubMed]
- Ulus IH, Wurtman RJ, Mauron C, et al. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res 1989;484:217-27. [Crossref] [PubMed]
- Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458-62. [Crossref] [PubMed]
- Nizri E, Hamra-Amitay Y, Sicsic C, et al. Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology 2006;50:540-7. [Crossref] [PubMed]
- Shytle RD, Mori T, Townsend K, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 2004;89:337-43. [Crossref] [PubMed]
- Kawaratani H, Tsujimoto T, Kitazawa T, et al. Innate immune reactivity of the liver in rats fed a choline-deficient L-amino acid- defined diet. World J Gastroenterol 2008;14:6655-61. [Crossref] [PubMed]
- Albright CD, Tsai AY, Mar M, et al. Choline availability modulates the expression of TGFb1 and cytoskeletal proteins in the hippocampus of developing rat brain. Neurochem Res 1998;23:751-8. [Crossref] [PubMed]
- Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 2010;33:301-11. [Crossref] [PubMed]
- Schmitz F, Heit A, Dreher S, et al. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol 2008;38:2981-92. [Crossref] [PubMed]
- Lim HK, Choi YA, Park W, et al. Phosphatidic acid regulates systemic inflammatory responses by modulating the Akt-mammalian target of rapamycin-p70 S6 kinase 1 pathway. J Biol Chem 2003;278:45117-27. [Crossref] [PubMed]
- Wu P, Feng L, Kuang SY, et al. Effect of dietary choline on growth, intestinal enzyme activities and relative expressions of target of rapamycin and eIF4E-binding protein2 gene in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2011;317:107-16. [Crossref]
- Mai K, Xiao L, Ai Q, et al. Dietary choline requirement for juvenile cobia, Rachycentron canadum. Aquaculture 2009;289:124-8. [Crossref]
- Shiau SY, Lo PS. Dietary choline requirements of juvenile hybrid tilapia, Oreochromis niloticus x O. aureus. J Nutr 2000;130:100-3. [Crossref] [PubMed]
- Reddy HRV, Ganapathi Naik M, Annappaswamy TS. Evaluation of the dietary essentiality of vitamins for Penaeus monodon. Aquac Nutr 1999;5:267-75. [Crossref]
- Kawashima K, Yoshikawa K, Fujii YX, et al. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci 2007;80:2314-9. [Crossref] [PubMed]
- Qiu Y, Peng Y, Wang J. Immunoregulatory role of neurotransmitters. Adv Neuroimmunol 1996;6:223-31. [Crossref] [PubMed]
- Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem 2004;89:1252-9. [Crossref] [PubMed]
- Budiño B, Cal RM, Piazzon MC, et al. The activity of several components of the innate immune system in diploid and triploid turbot. Comp Biochem Physiol A Mol Integr Physiol 2006;145:108-13. [Crossref] [PubMed]
- Zapata A, Diez B, Cejalvo T, et al. Ontogeny of the immune system of fish. Fish Shellfish Immunol 2006;20:126-36. [Crossref] [PubMed]
- Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004;131:1619-28. [Crossref] [PubMed]
- Tocher DR, Bendiksen EÅ, Campbell PJ, et al. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008;280:21-34. [Crossref]
- Zeisel SH. Choline phospholipids: signal transduction and carcinogenesis. FASEB J 1993;7:551-7. [Crossref] [PubMed]
- Kortstee GJ. The aerobic decomposition of choline by microorganisms I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as the sole carbon and nitrogen source. Arch Mikrobiol 1970;71:235-44. [Crossref] [PubMed]
- Niizeki N, Daikoku T, Hirata T, et al. Mechanism of biosynthesis of trimethylamine oxide from choline in the teleost tilapia, Oreochromis niloticus, under freshwater conditions. Comp Biochem Physiol B Biochem Mol Biol 2002;131:371-86. [Crossref] [PubMed]
Cite this article as: Al-Humadi A, Al-Humadi H, Liapi C. Novel insight on the impact of choline-deficiency in sepsis. Ann Res Hosp 2019;3:12.


